Compare the SIR effect between orthochloro benzoic acid, orthobromobenzoic acid and orthoiodo benzoic acid.
Video Solution
Text Solution
The correct Answer is:SIR effect increases with the size of ortho group. The order of SIR effect is o-iodo benzoic acid >o− bromo benzoic acid >o− chloro benzoic acid.
|
Answer
Step by step video, text & image solution for Compare the SIR effect between orthochloro benzoic acid, orthobromobenzoic acid and orthoiodo benzoic acid. by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Knowledge Check
Benzoic acid not form in :
A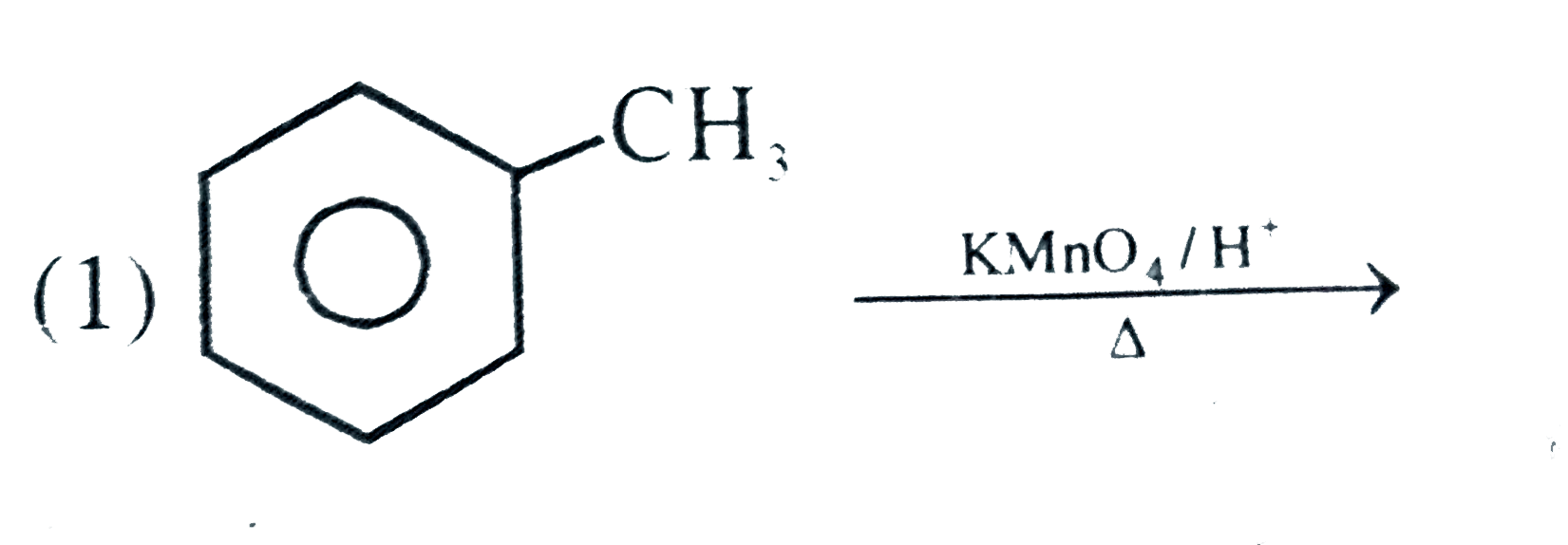 B
B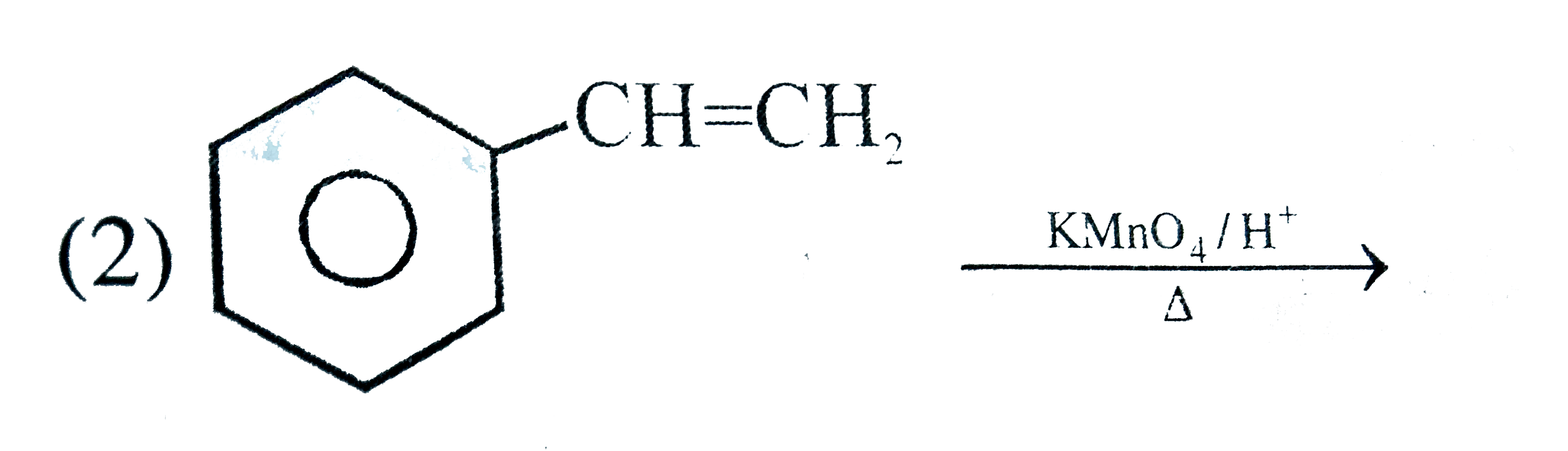 C
C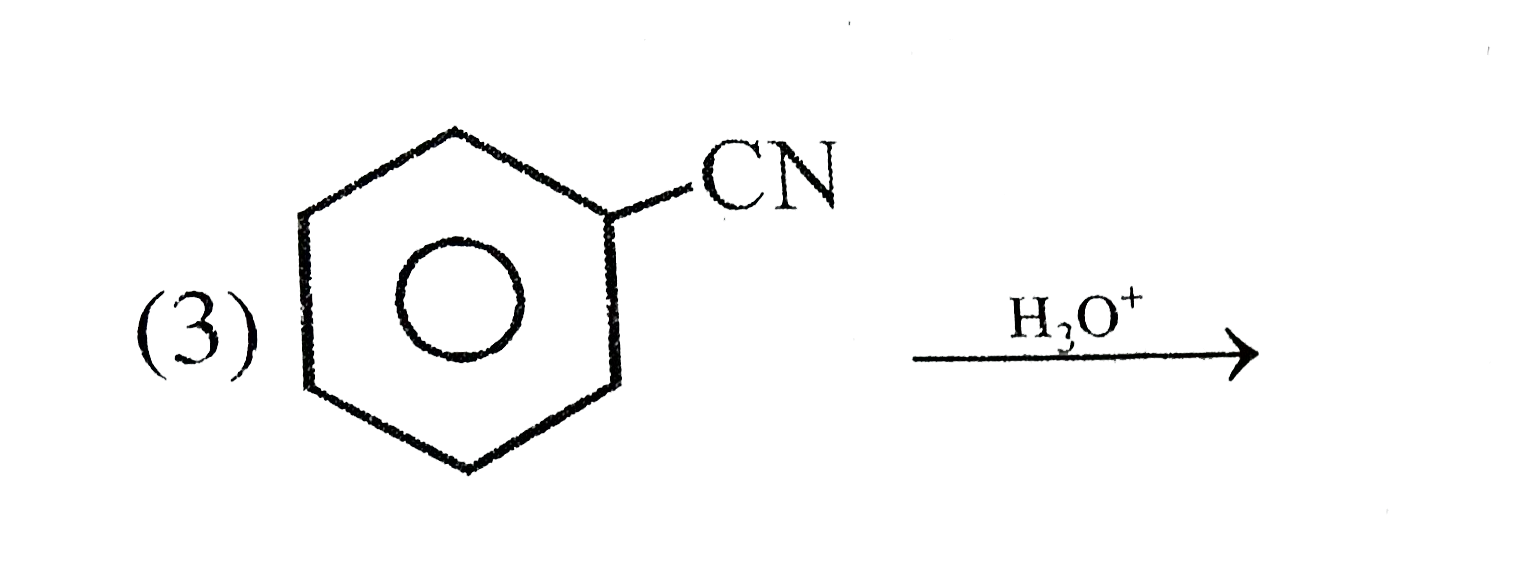 D
D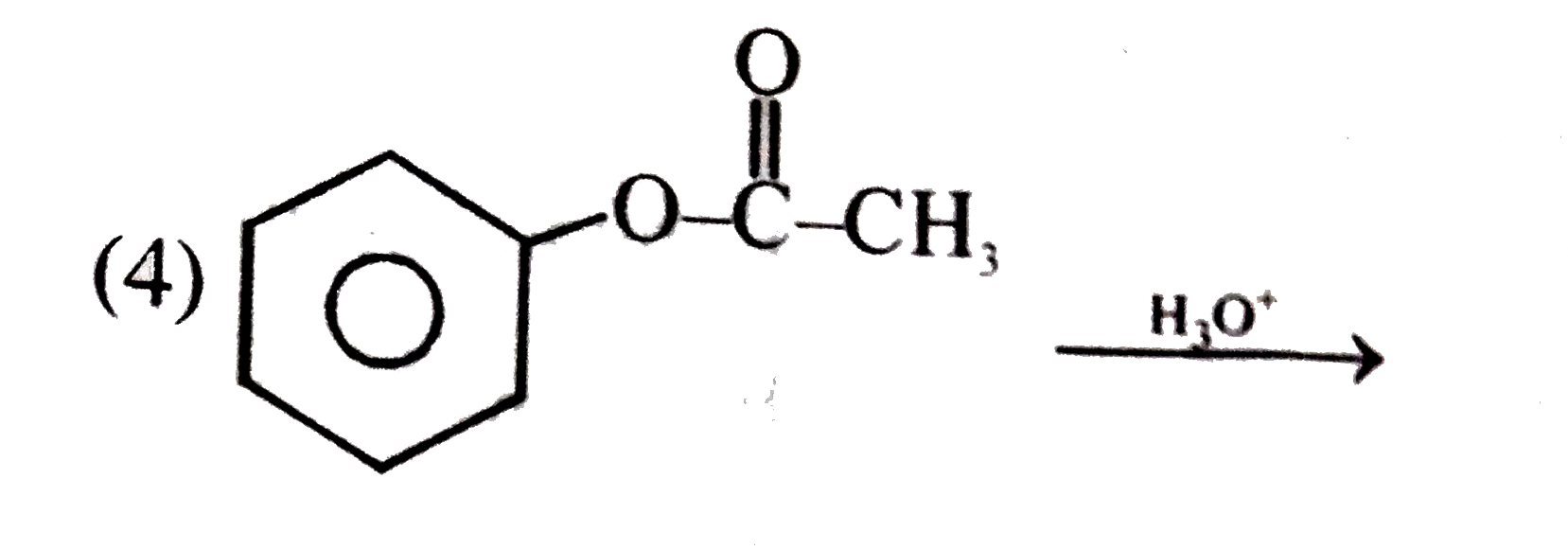
Benzoic acid contains
A15σand2π- bondsB15σand4π- bondsC14σand4π - bondsD13σand4π- bondsBenzoic acid can be purified by
ASublimationBCrystallizationCDistillationDChromatography

