The lone pair of amines makes them basic. They react with acids to form acid-base salts. Amines are more basic than alcohols, ethers and water. When an amine is dissolved in water, an equilibrium is established , where water acts as an acid and transfer a proton to the amine. The basic strength of an amine can be measured by basicity constant Kb.
Arylamines are less basic than alkylamines because the lone pair of nitrogen is delocalised with the aromatic ring and are less available for donation.
Substituted arylamines can be either more basic or less basic than aniline , depending on the substituted . ERG substituents, such as −CH3,−NH2and−OCH3 increases the basicity and EWG substituents , such as −Cl,−NO2and−CN decreases basicity. While sp2− hybridized nitrogen atom in pyridine is less basic then the sp3 -hybridized nitrogen in an alkylamine.
Select the correct order of Kb
CH3NH2>NaOH
Pyridine >CH3−NH−CH3
p-Methyl aniline > p-Chloroaniline > p-Amino acetophenone
p-Bromoaniline > p-Nitroaniline > p-Amino benzaldehyde
The correct Answer is:C
Topper's Solved these Questions
- GENERAL ORGANIC CHEMISTRY IIBook:RESONANCEChapter:GENERAL ORGANIC CHEMISTRY IIExercise:Exercise-3 Part:I JEE(Advanced)/IIT-JEE Problems (Previous Years)
Explore 17 Videos
- GENERAL ORGANIC CHEMISTRY IIBook:RESONANCEChapter:GENERAL ORGANIC CHEMISTRY IIExercise:Exercise-3 Part:II JEE(MAIN)/AIEEE PROBLEMS (PREVIOUS YEARS)
Explore 18 Videos
- GENERAL ORGANIC CHEMISTRY IIBook:RESONANCEChapter:GENERAL ORGANIC CHEMISTRY IIExercise:Part-III: One or More Than One Options Correct Type
Explore 9 Videos
- GASEOUS STATEBook:RESONANCEChapter:GASEOUS STATEExercise:PHYSICAL CHMISTRY (Gaseous State)
Explore 47 Videos
- GENERAL ORGANIC CHEMISTRY-IBook:RESONANCEChapter:GENERAL ORGANIC CHEMISTRY-IExercise:PART-III : PRACTICE TEST-19
Explore 1 Video
Similar Questions
The lone pair of amines makes them basic. They react with acids to form acid-base salts. Amines are more basic than alcohols, ethers and water. When an amine is dissolved in water, an equilibrium is established , where water acts as an acid and transfer a proton to the amine. The basic strength of an amine can be measured by basicity constant Kb.
Arylamines are less basic than alkylamines because the lone pair of nitrogen is delocalised with the aromatic ring and are less available for donation.
Substituted arylamines can be either more basic or less basic than aniline , depending on the substituted . ERG substituents, such as −CH3,−NH2and−OCH3 increases the basicity and EWG substituents , such as −Cl,−NO2and−CN decreases basicity. While sp2− hybridized nitrogen atom in pyridine is less basic then the sp3 -hybridized nitrogen in an alkylamine.
The most basic carbanion is :
View SolutionThe lone pair of amines makes them basic. They react with acids to form acid-base salts. Amines are more basic than alcohols, ethers and water. When an amine is dissolved in water, an equilibrium is established , where water acts as an acid and transfer a proton to the amine. The basic strength of an amine can be measured by basicity constant Kb.
Arylamines are less basic than alkylamines because the lone pair of nitrogen is delocalised with the aromatic ring and are less available for donation.
Substituted arylamines can be either more basic or less basic than aniline , depending on the substituted . ERG substituents, such as −CH3,−NH2and−OCH3 increases the basicity and EWG substituents , such as −Cl,−NO2and−CN decreases basicity. While sp2− hybridized nitrogen atom in pyridine is less basic then the sp3 -hybridized nitrogen in an alkylamine.
pKb order of the following compound is :
(I) NH2OH (II)NH2NH2 (III)NH3 (IV)H2O
View Solution
Knowledge Check
Amines are more basic than
AAlcoholsBNaOHCLiOHDKOHAmines are more basic than
AalcoholsBethersCestersDAll of theseThe lone pair of amines makes them basic. They react with acids to form acid-base salts. Amines are more basic than alcohols, ethers and water. When an amine is dissolved in water, an equilibrium is established , where water acts as an acid and transfer a proton to the amine. The basic strength of an amine can be measured by basicity constant Kb.
Arylamines are less basic than alkylamines because the lone pair of nitrogen is delocalised with the aromatic ring and are less available for donation.
Substituted arylamines can be either more basic or less basic than aniline , depending on the substituted . ERG substituents, such as −CH3,−NH2and−OCH3 increases the basicity and EWG substituents , such as −Cl,−NO2and−CN decreases basicity. While sp2− hybridized nitrogen atom in pyridine is less basic then the sp3 -hybridized nitrogen in an alkylamine.
The most basic carbanion is :A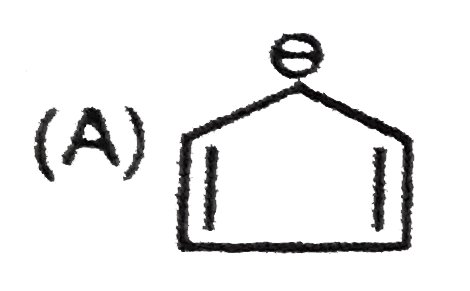 BCH≡ΘCC
BCH≡ΘCC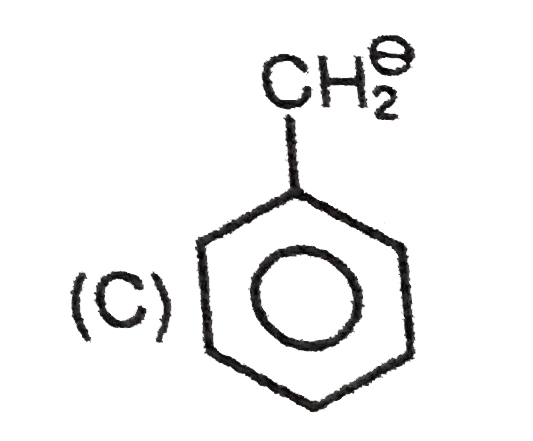 D
D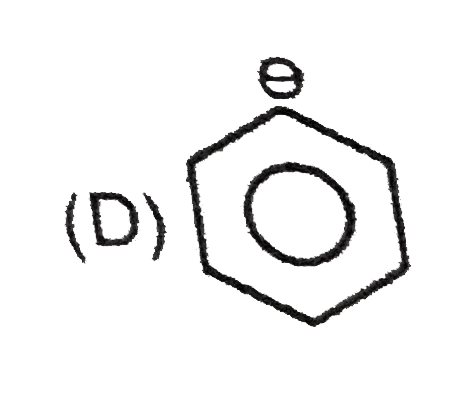
Amines are more basic than
View SolutionThe lone pair of amines makes them basic. They react with acids to form acid-base salts. Amines are more basic than alcohols, ethers and water. When an amine is dissolved in water, an equilibrium is established , where water acts as an acid and transfer a proton to the amine. The basic strength of an amine can be measured by basicity constant Kb.
Arylamines are less basic than alkylamines because the lone pair of nitrogen is delocalised with the aromatic ring and are less available for donation.
Substituted arylamines can be either more basic or less basic than aniline , depending on the substituted . ERG substituents, such as −CH3,−NH2and−OCH3 increases the basicity and EWG substituents , such as −Cl,−NO2and−CN decreases basicity. While sp2− hybridized nitrogen atom in pyridine is less basic then the sp3 -hybridized nitrogen in an alkylamine.
pKb order of the following compound is :
(I) NH2OH (II)NH2NH2 (III)NH3 (IV)H2O
View SolutionTrimethyl amine is less basic than methyl amine because
View SolutionTrimethyl amine is less basic than methyl amine because
View SolutionAryl amines are less basic than alkyl amine and ammonia which is due to
View Solution

