The carboxyl functional group (-COOH) is present in
picric acid
barbituric acid
ascorbic acid
aspirin
The correct Answer is:D
Topper's Solved these Questions
- GENERAL ORGANIC CHEMISTRY IIBOOK - RESONANCECHAPTER - GENERAL ORGANIC CHEMISTRY IIEXERCISE - Exercise-3 Part:II JEE(MAIN)/AIEEE PROBLEMS (PREVIOUS YEARS)
18 Videos
- GENERAL ORGANIC CHEMISTRY IIBOOK - RESONANCECHAPTER - GENERAL ORGANIC CHEMISTRY IIEXERCISE - Exercise-3 Part:II JEE(MAIN) ONLINE PROBLEMS
16 Videos
- GENERAL ORGANIC CHEMISTRY IIBOOK - RESONANCECHAPTER - GENERAL ORGANIC CHEMISTRY IIEXERCISE - Part-IV : Comprehension
14 Videos
- GASEOUS STATEBOOK - RESONANCECHAPTER - GASEOUS STATEEXERCISE - PHYSICAL CHMISTRY (Gaseous State)
47 Videos
- GENERAL ORGANIC CHEMISTRY-IBOOK - RESONANCECHAPTER - GENERAL ORGANIC CHEMISTRY-IEXERCISE - PART-III : PRACTICE TEST-19
1 Video
Similar Questions
The correct order of increasing basic nature for the bases NH3CH3NH2and(CH3)2NH is :
View SolutionIn which of the following pairs A is more stable than B ?
View SolutionReaction intermdiates are short lived species and are highly reactive. They are formed by heterolytic and homolytic bond fission. There are various types of reaction intermediates in which the most important are carbocation , carbanion and free radical.
Carbocation is an organic species in which carbon have positive charge and six electrons in its outermost shell. The stability of carbocation can be increased by positive inductive effect, hyperconjugation and delocalisation. If α-atom with respect to carbocation has one or more lone pair of electron then lone pair of electron strongly stabilises the carbocation due to octet completion.
Species in which carbon have negative charge is called carbanion. Carbanion carries three bond pairs and one lone pair. The stability of carbanion can be increased by negative inductive effect, negative mesomeric effect and delocalisation.
Free radical is a species which have seven electrons in its outermost shell. The stability of free radical can be increased by hyperconjugation and delocalisation.
Which of the following is the most stable carbanion intermediate ?
View SolutionA certain quantity of a gas occupied 100ml when collected over water at 15∘C and 750mm pressure . It occupies 91.9ml in dry state at NTP. Find the V.P. of water at 15∘C
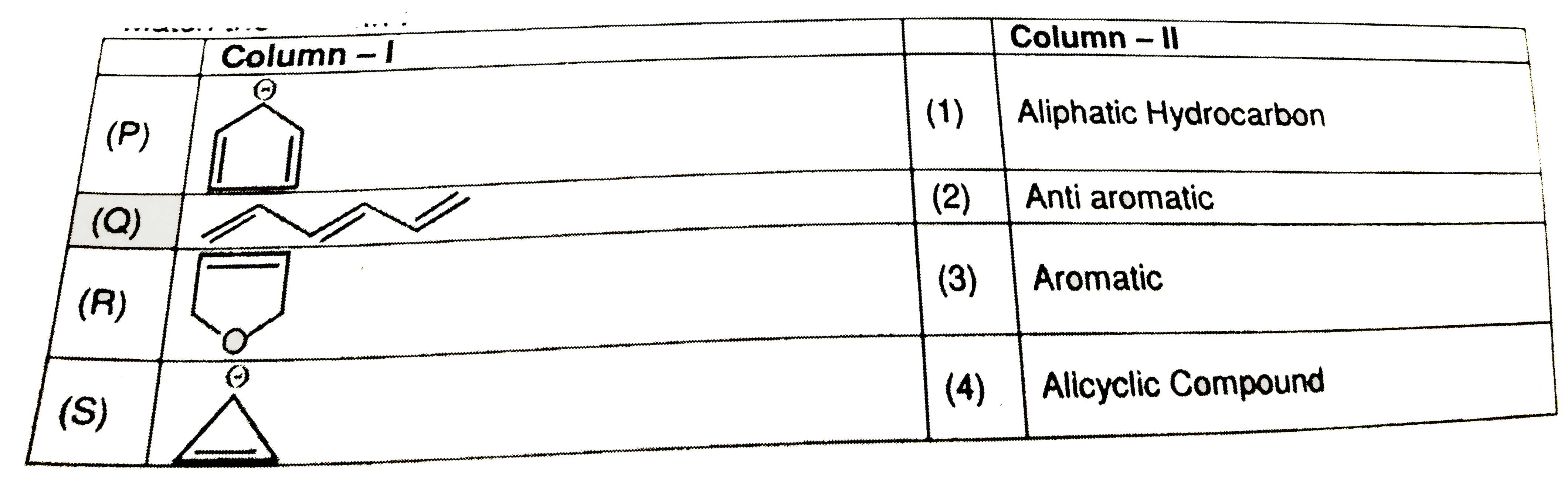
View SolutionMatch the column.
View SolutionThe carboxyl functional group (−COOH) is present in :
View SolutionThe carboxyl functional group(−COOH) is present in:
View SolutionThe carboxylic functional group (-COOH) is present in :
View SolutionThe carboxyl functional group (-COOH) is present in
View SolutionThe carboxyl functional group (-COOH) is present in
View SolutionName the functional group present in carboxylic acids.
View SolutionThe carboxyl functional group (-COOH) is present in
View Solutionकार्बोक्सिल क्रियात्मक समूह (-COOH) उपस्थित होता है :
View Solution−COOHक्रियात्मक समूह कार्बोक्सिलिक अम्ल का नहीं है।
View SolutionThe functional group present in carboxylic acids is :
View Solution

