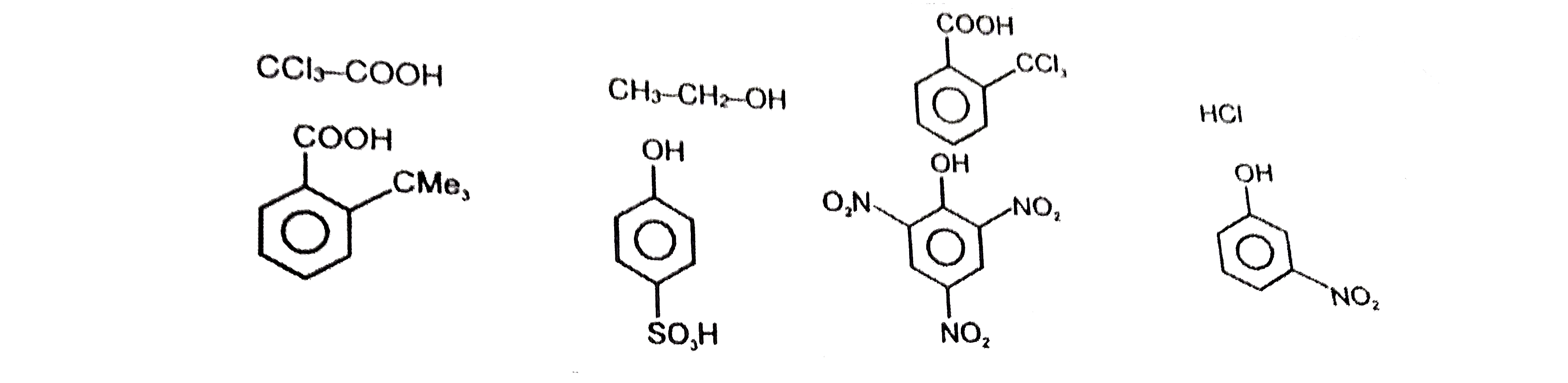
How many of the following compounds react with NaHCO3 and liberate CO2(g)
1. Salicylic acid 2. Pthalic acid 3. Picric acid 4.Resorcinol
5. Carbolic acid 6.Aspirin 7.Anisol 8.Tarteric acid
The correct Answer is:5(1,2,3,6,8)
Topper's Solved these Questions
Similar Questions
Knowledge Check
some of the following acids lack -COOH group but liberate CO2 with NaHCO3. These acids are
AAscorbic acidBPicric acidCCarbolic acidDSalicylic acidA : Phenol does not react with NaHCO3 .
R : Phenol is less acidic than H2CO3AIf both Assertion & Reason are true and the reason is the correct explanation of the assertion, then mark (1)BIf both Assertion & Reason are true but the reason is not the correct explanation of the assertion, then mark (2)CIf Assertion is true statement but Reason is false , then mark (3)DIf both Assertion and Reason are false statements , then mark (4)Sodium phenoxide react with CO2 at 125∘C under 5 atm pressure to give salicylic acid. This reaction is called
AKolbe's reactionBPerkin reactionCWurtz reactionDHVZ reaction
Amongst the following how many compounds are more acidic than benzoic acid ?
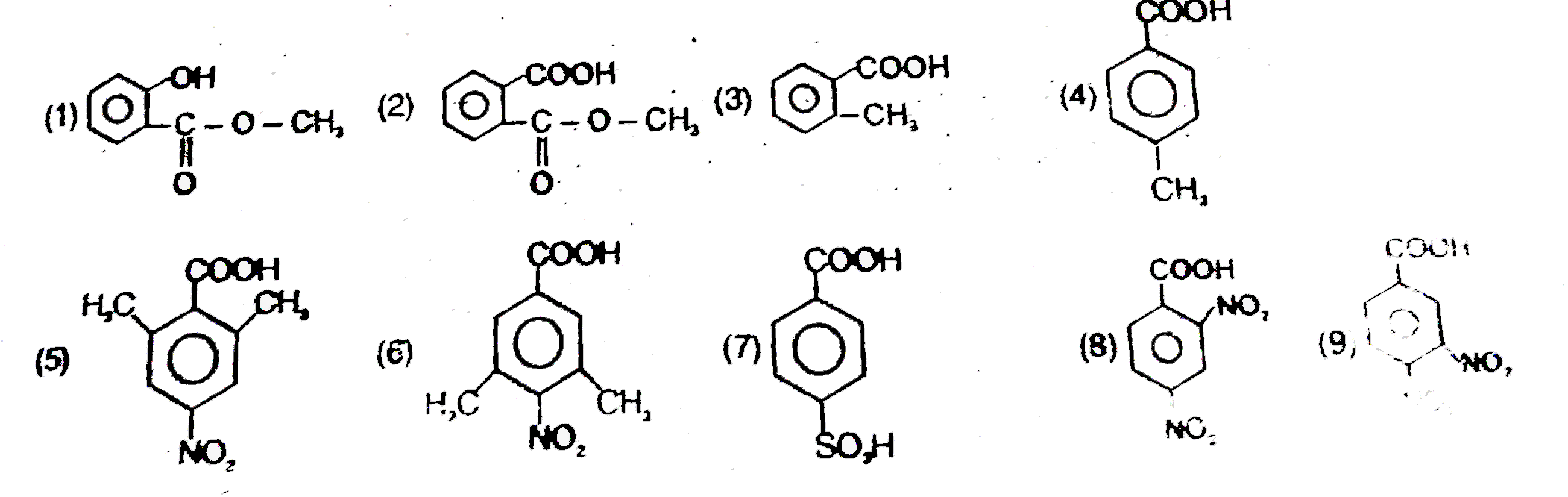
View SolutionHow many of the following contains carboxylic acid group?
Picric acid, salicylic acid, adipic acid, saccharin.
View SolutionHow many of the following compounds will change blue litmus into red ?
(a) Ethanol (b) Ethanoic acid
( c) Ethanal (d) Propanone
(e ) Phenol (f) Squaric acid
(g) Propyne (h)Picric acid
View SolutionHowmany of the following compounds are dissolve in aqeous solution of NaOH
(1) Phenol (2) p-cresol (3) Benzoic acid (4) Benzene sulphonic acid (5) Aniline (6) P-toluedine (7) Picric acid (8) Squaric acid
View SolutionRank the following compounds in increasing order of their acidity:
1. 3 - fluorobutanoic acid
2. 3 - chlorobutanoic acid
3. 2 - flurobutanoic acid
4. Butanoic acid
5. 4 - chlorobutanoic acid.
View Solution