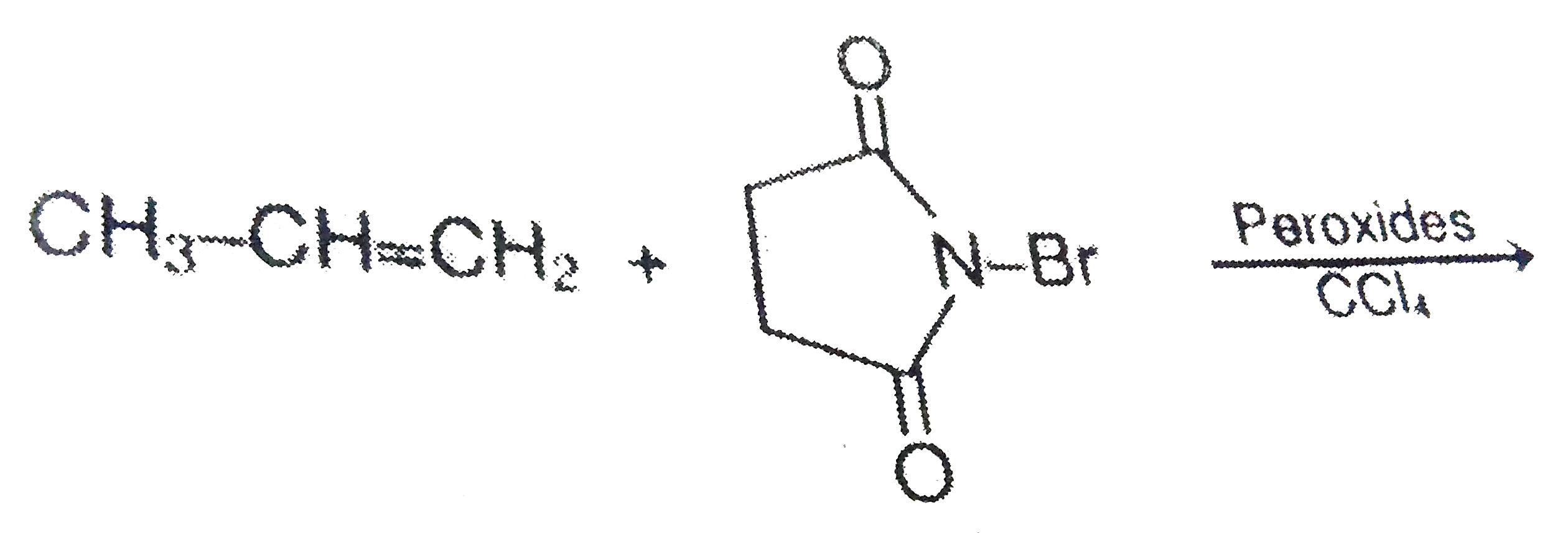
This reaction is classified as
A
Electrophilic substitution reaction
B
Free radical substitution reaction
C
Nucleophilic substitution reaction
D
Electrophilic addition reaction
Text Solution
The correct Answer is:B
|
Similar Questions
Knowledge Check
CH2=CHOH→CH3−O∣∣C−H
The above reaction is classified as _______ reaction.ASubstitutionBAdditionCEliminationDRearrangementThe following reaction is classified as :
CH3CH2I+KOH(aq)→CH3CH2OH+KIAelectrophilic substitutionBnucleophilic substitutionCeliminationDadditionR−X+NaOH→ROH+NaX
The above reaction is classified asANucleophilic substitutionBElectrophilic substitutionCReductionDOxidation
The reaction
can be classified as
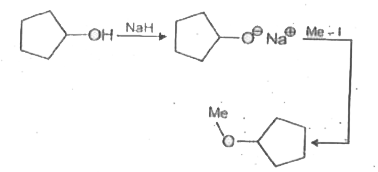
View SolutionThe reaction
can be classified as

View SolutionCH3−Br+NH3→CH3−NH2+HBr
The above reaction is classified as
View SolutionR-X + NaOH → ROH + Na X, The above reaction is classified as:
View SolutionCalcium oxide reacts vigorously with water to produce slaked lime.
CaO (s)+H2O (I)→Ca(OH)2 (aq)
This reaction can be classified as :
(A) Combination reaction
(B) Exothermic reaction
(C) Endothermic reaction
(D) Oxidation reaction
View Solution

