The reaction of one equivalent of HBr with CH2=CH−CH2−C≡CH gives :
A
CH2=CH−CH2−C≡CBr
B
CH2=CH−CH2−C∣Br=CH2
C
CH3−C∣BrH−CH2−C≡CH
D
CH2=CH−CH2−CH=CHBr
Text Solution
The correct Answer is:C
|
Similar Questions
Knowledge Check
The reaction of one equivalent of HBr with
CH2=CH−C≡CH givesACH2=CH−C≡CBrBCH2=CH−CBr=CH2CCH3−CHBr−C≡CHDCH3−CHBr−C≡CHAddition of 1 equivalent of HCL to CH2=C=CH2 gives
A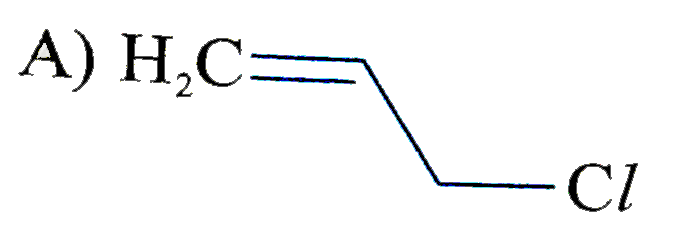 B
B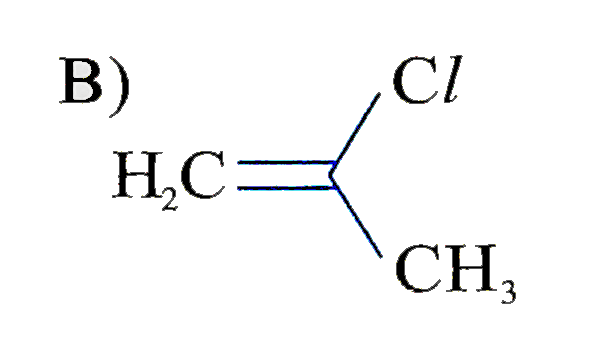 C
C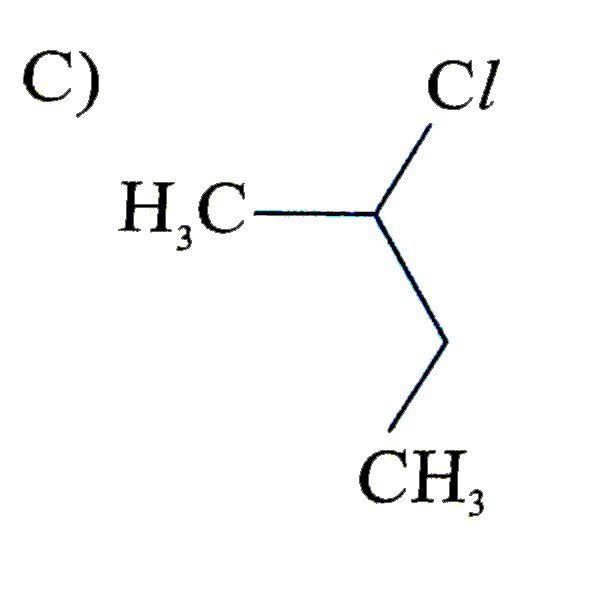 D
D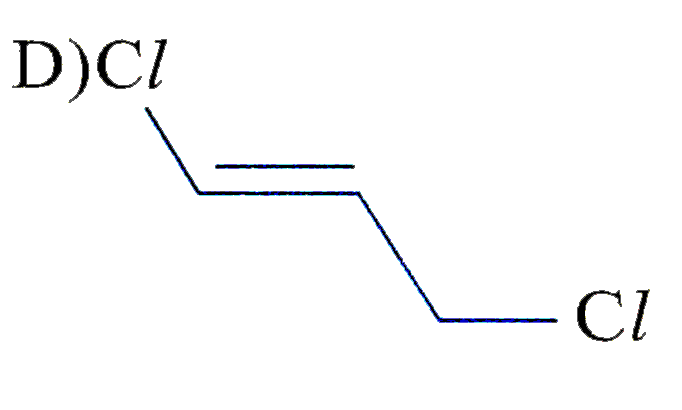
The reaction of CH3CH=
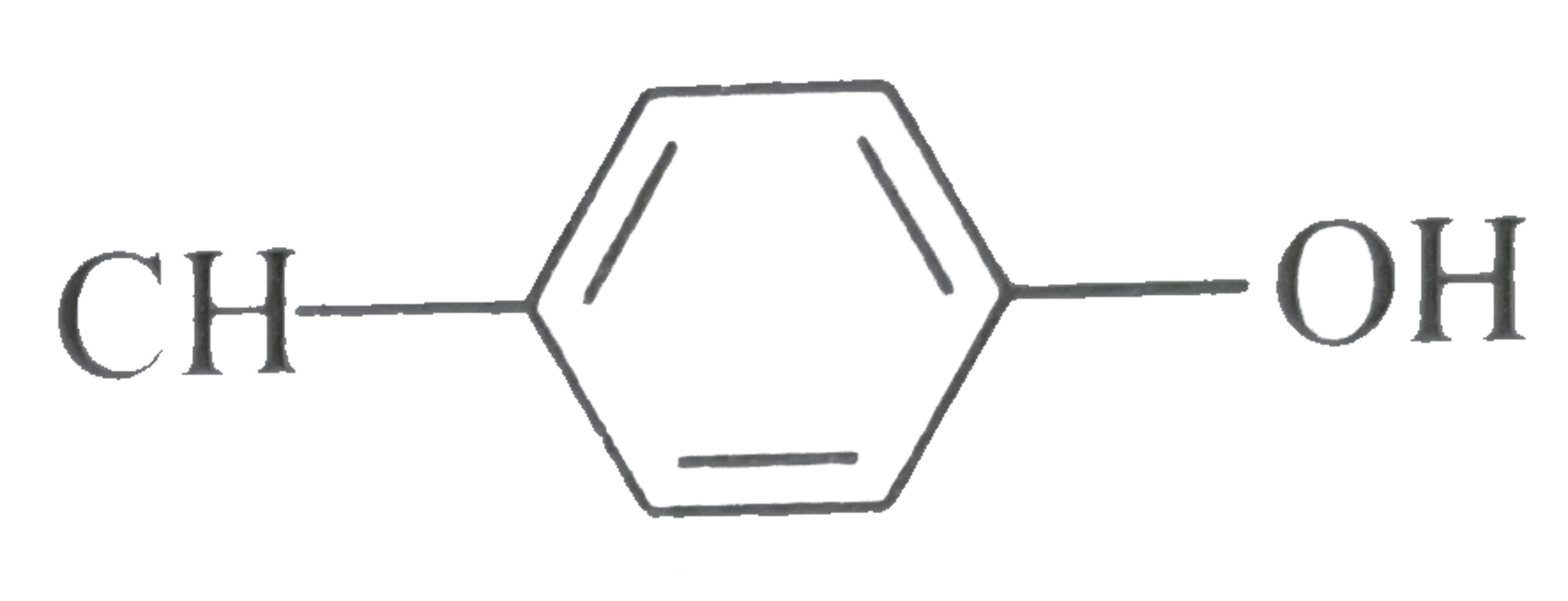 with HBr givesA
with HBr givesA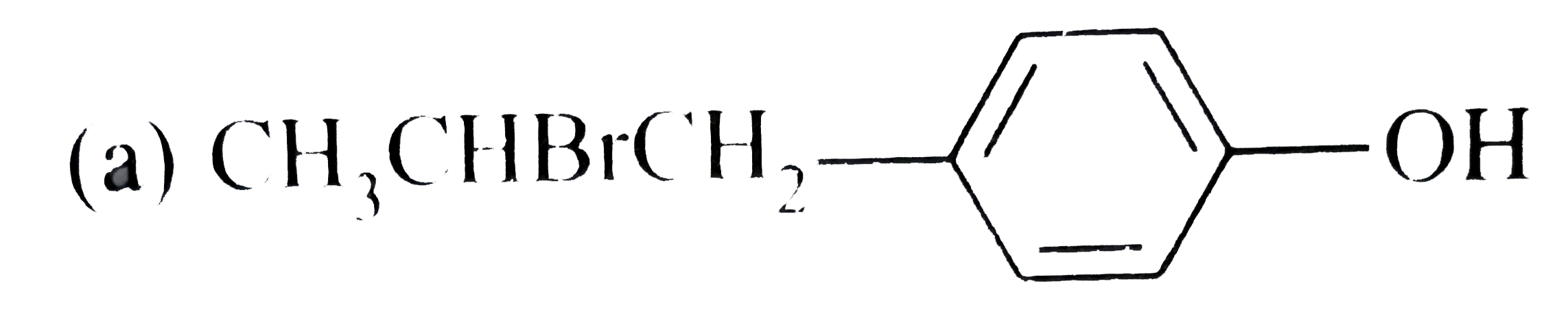 B
B C
C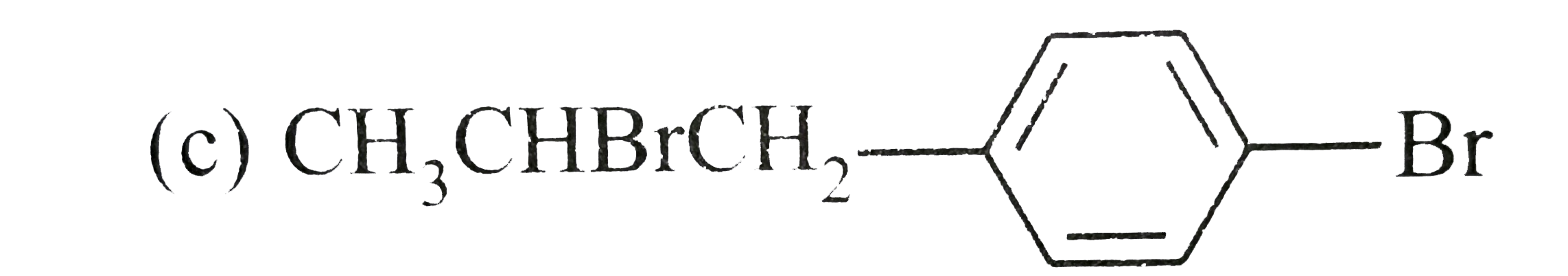 D
D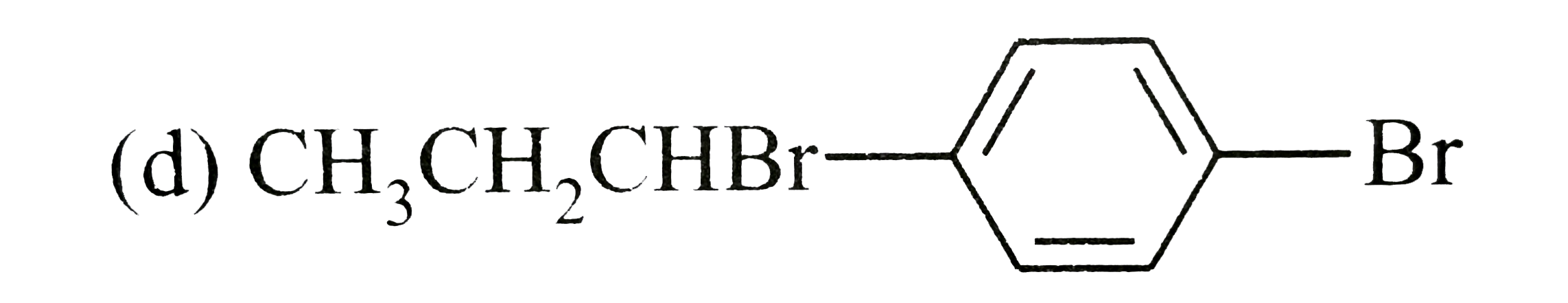
Predict the product of the reaction given below :
CH3−CH2−CH=CH2HBr−−−−−−−−−→Peroxide or light
View SolutionThe reaction of CH3CH=CH−
 with HBr gives :
with HBr gives :
View SolutionThe reaction of HBr with CH3C(CH3)=CH2 in the presence of peroxide will give
View SolutionThe reaction of HBr with CH3C∣CH3=CH2 in the presence of peroxide will give
View SolutionThe compound , which on reductive ozonolysis gives one mole of O=CH−CH2−CH2−CH2−CH=O, is
View Solution

