A Hydrocarbon X (M.F.C4H6) produces an aldehyde Y through Hydrocarbon Oxidation and a ketone Z through Oxymercuration Demercuration. Y and Z are functional isomers. X gives P when treated with excess of HOCl and Q when treated with excess of HCl. 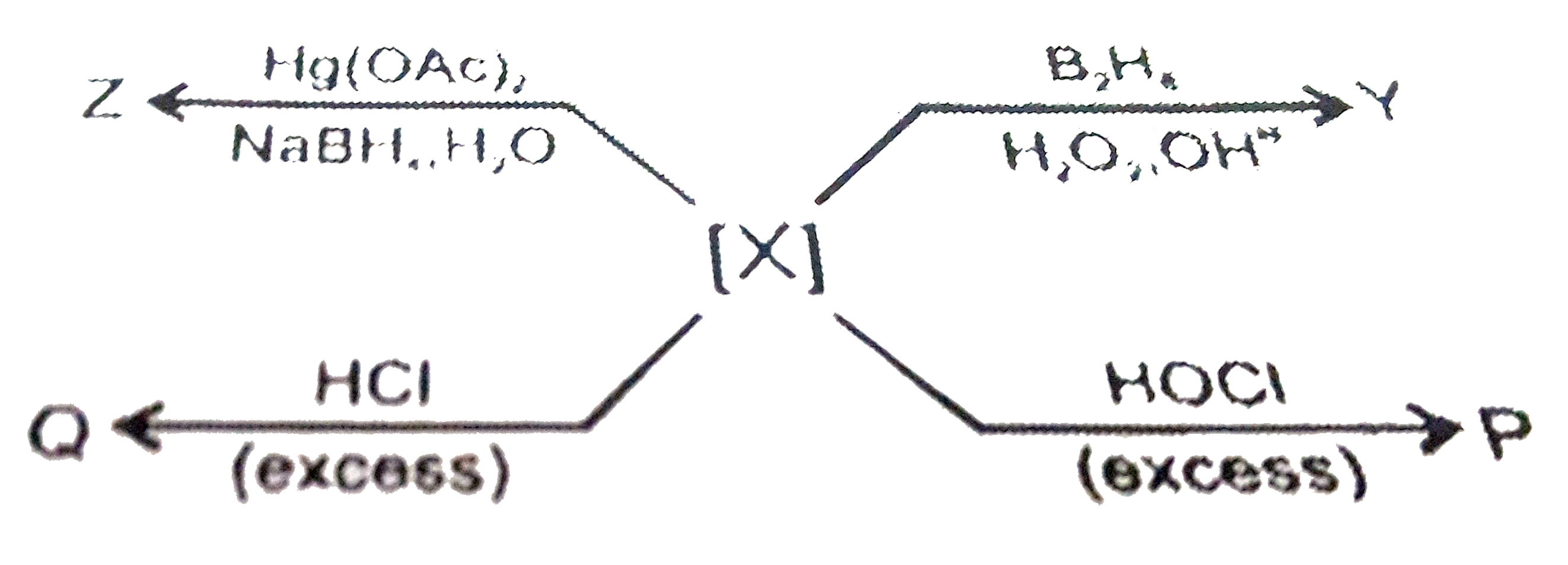
The structure of X is :
CH3−C≡C−CH3
CH3−CH2C≡CH
CH2=CH−CH=CH2
CH2=CH−CH≡CH
The correct Answer is:B
Similar Questions
When phenol in treated with excess bromine water, it gives
View SolutionPhenol treated with excess of bromine water gives _____.
View SolutionWhen phenol is treated with excess of bromine water, it gives
View SolutionWhen phenol is treated with excess of bromine water, it gives
View SolutionA Hydrocarbon X (M.F.C4H6) produces an aldehyde Y through Hydroboration Oxidation and a ketone Z through Oxymercuration Demercuration. Y and Z are functional isomers. X gives P when treated with excess of HOCl and Q when treated with excess of HCl.
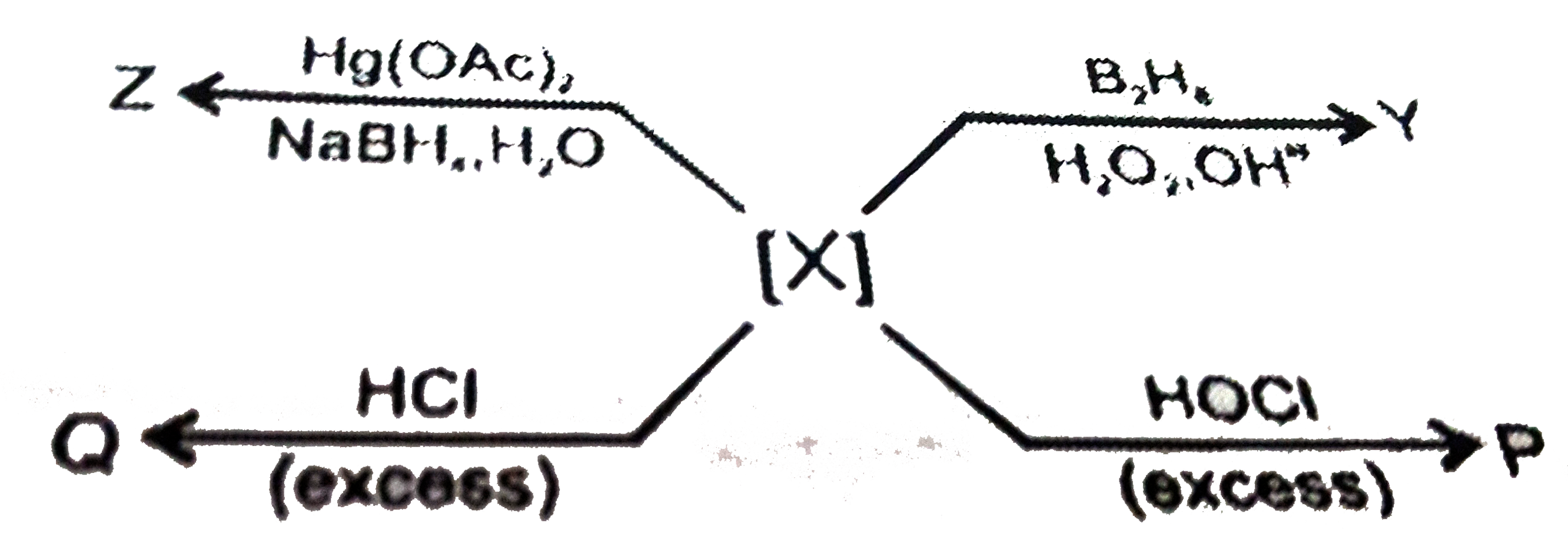
The correct statement is :
View SolutionWhen tetrahydrafuran is treated with excess HI, the product formed is
View SolutionWhen glycerol is treated with excess of HI, it produces
View SolutionA red coloured mixed oxide (X) on treatment with conc. HNO3 gives a compound (Y). (Y) with HCl produces a chloride (Z) which is insoluble in cold water but soluble in hot water, (Z) can also be formed by treating (X) with conc. HCl. Compounds (X), (Y) and (Z) are :
View Solution

