Rate of Bimolecular elimination (E2) reaction for the following :
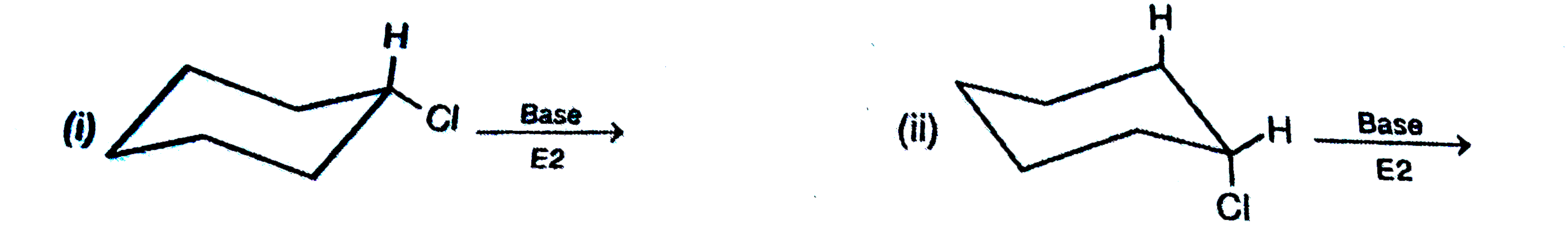
Same for both conformers
I gt II
ii gt I
Can't say anything about rate of E-2 reaction
The correct Answer is:C
The more stable conformation of chlorocyclohexane does not undergo an E-2 elimination easily while the less stable conformer , with the chloro group in the axial position , readily undergoes an E-2 reaction.
Similar Questions
Knowledge Check
The following bimolecular eleimination reaction (E2)is carried out with different halogen leaving groups the cent yield of the two product (2-hexene and 1-hexene ) for each leacing group is lested below .

which of the following statement is (are) true concerning this seris of E2 reaction ?ABased on the Pka s of the the conjugate acid I− is the best leaving group and F− is the poorest leaving groupBWhen I−Br−andCl− aarae used as leaving group , Zaitsev 's rule is gollowdCF−is the stogest base (and therefore the poorest lec=ving group) an dthe transition state for reaction with fluorde as the the leaving f=group has the double bond charasterDa,b,c are true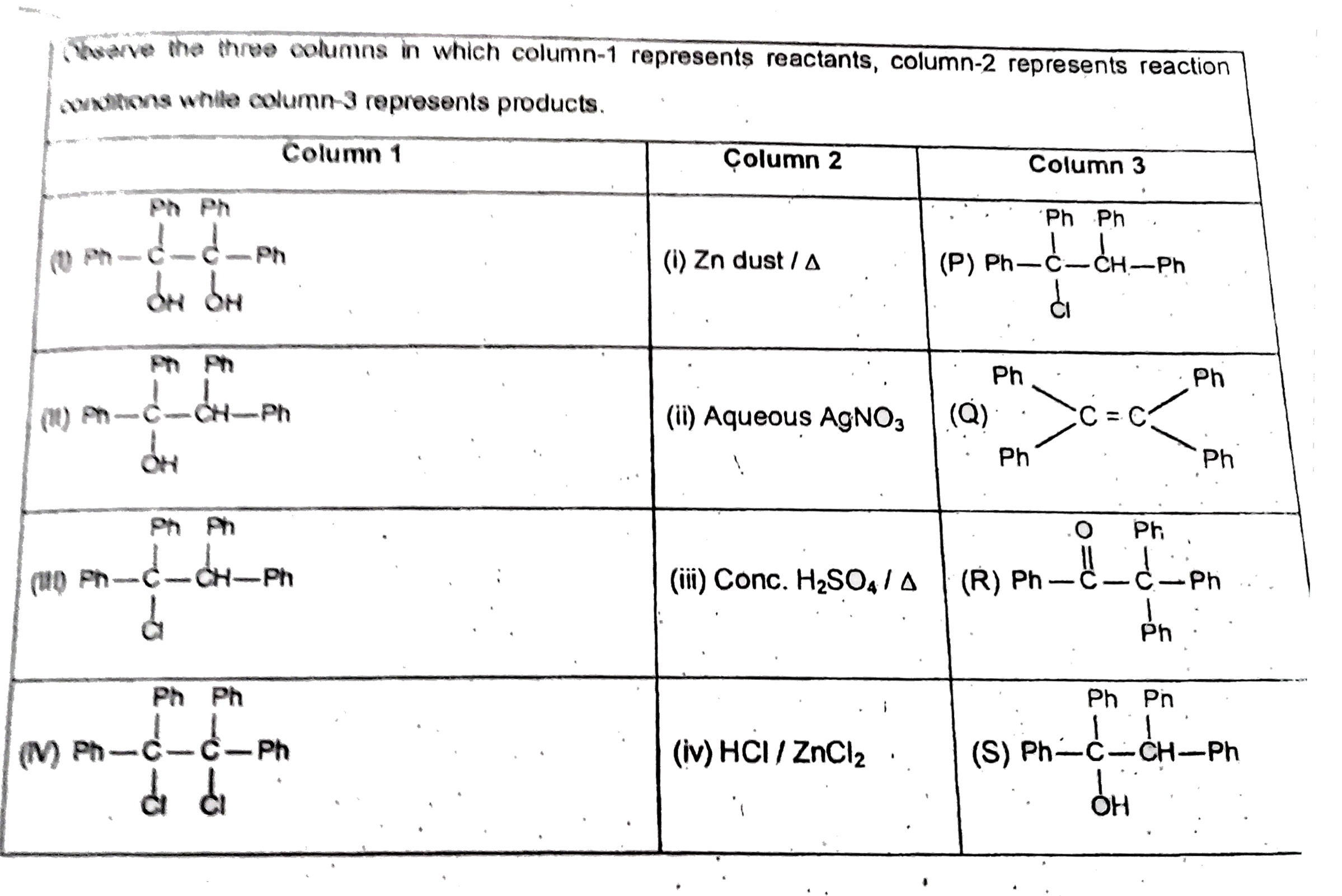
The bimolecular reaction is represented by :A(I)(ii)(R)B(IV)(i)(Q)C(III)(ii)(S)D(II)(iv)(P)
The bimolecular reaction is represented by :A(IV),(iii),(Q)B(I),(ii),(S)C(III),(ii),(P)D(II),(i),(R)
|| E2 and E1 elimination reaction ||
View SolutionComparision Between Unimolecular Elimination(E1) & Dimolecular Elimination(E2) | Comparision Between Elimination & Substitution | E1cB Mechanism | Ei Mechanism | Hoffmann Exhaustive Methylation
View SolutionE2 Elimination Reaction (Dehydrohalogenation)
View SolutionAlkyl halides undergo elimination when heated with strong base. A concerted reaction in which both the groups to be eliminated, leave silumtaneously is E2 reaction. In another case ionization of alkyl halide takes place losing X− and forms carbocation in the first stage followed by the loss of H+ . this is substituted alkene) and Hofmann (less substituted alkene) are formed depending on the condition of the reaction.
Q. Which of the following is not correct regarding E2 reaction?
View SolutionWhich of the following is β-elimination reaction ?
View Solution

