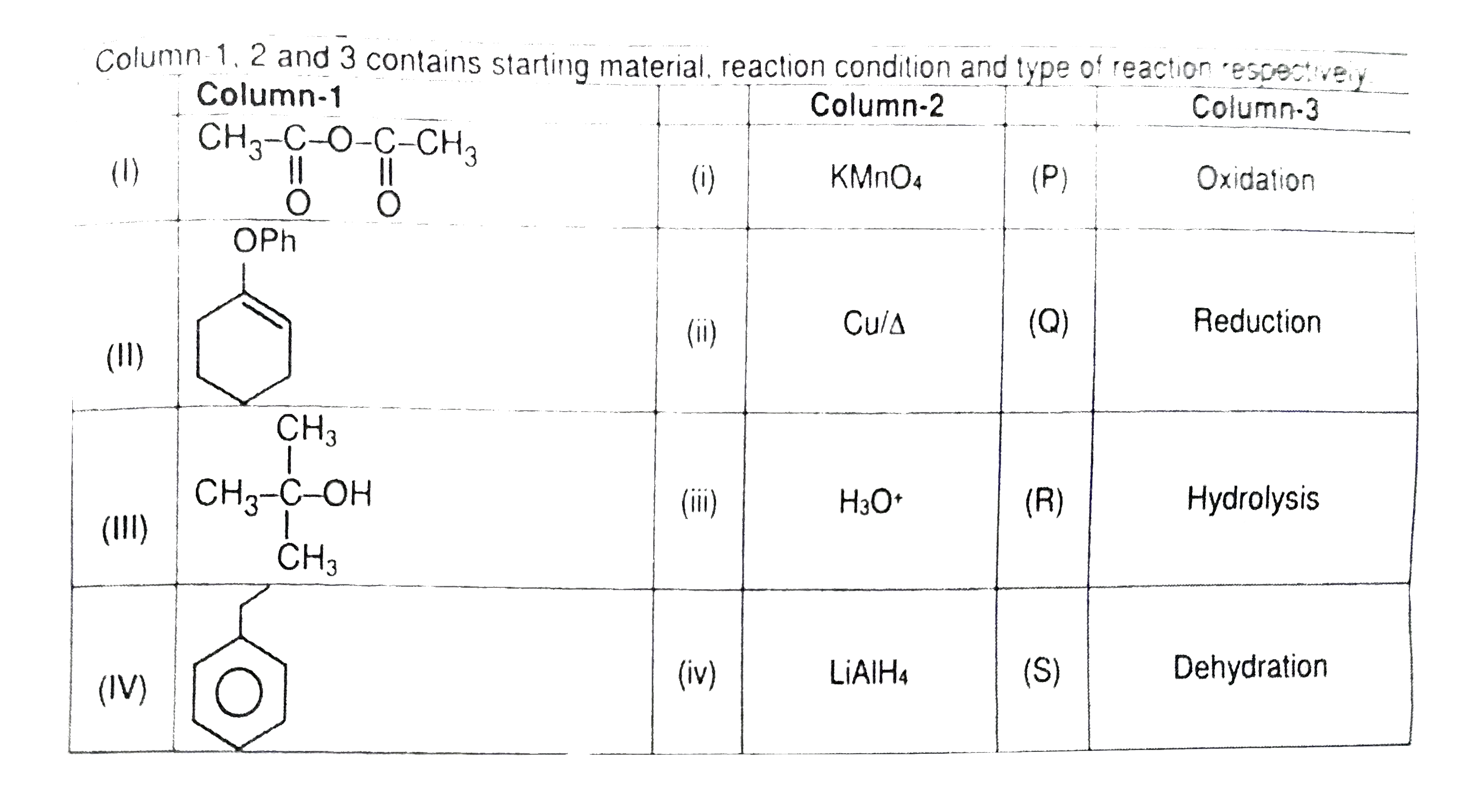
Which of the combination of reaction result in formation of an alkene.
A
(I) (iv) Q
B
(III) (i) S
C
(III) (i) S
D
(II), (iii) R
Text Solution
The correct Answer is:C
|
Topper's Solved these Questions
- REDUCTION, OXIDATION & HYDROLYSIS REACTIONSBook:RESONANCEChapter:REDUCTION, OXIDATION & HYDROLYSIS REACTIONSExercise:Part-I : Jee (Advanced) (Previous years)
Explore 13 Videos
- REDUCTION, OXIDATION & HYDROLYSIS REACTIONSBook:RESONANCEChapter:REDUCTION, OXIDATION & HYDROLYSIS REACTIONSExercise:Part-II JEE (Main) AIEEE Problems (Previous Years)
Explore 16 Videos
- REDUCTION, OXIDATION & HYDROLYSIS REACTIONSBook:RESONANCEChapter:REDUCTION, OXIDATION & HYDROLYSIS REACTIONSExercise:Part-III: One or more than one options correct type
Explore 5 Videos
- PERIODIC TABLE & PERIODICITYBook:RESONANCEChapter:PERIODIC TABLE & PERIODICITYExercise:Inorganic chemistry (Periodic Table & Periodicity
Explore 61 Videos
- S BLOCK ELEMENTSBook:RESONANCEChapter:S BLOCK ELEMENTSExercise:INORGANIC CHEMISTRY(s-Block Elements)
Explore 36 Videos
Similar Questions
Knowledge Check
Which of the following combination of sides results in the formation of obtuse angled triangle?
A6,7,13B5, 6, 8C4, 5, 6DNone of theseWhich of the following combination of sides results in the formation of obtuse angled triangle?
A6, 7, 13B5, 6, 8C4, 5, 6,DNone of theseWhich of the following reactions can result in formation of a colloidal solution?
AReduction of SO2(g) with H2S to give sulphur and water.BReduction of AuCl3 with HCHO in presence of water.CHydrolysis of FeCl3 in presence of NaOH.DHydrolysis of ester in presence of acid.
Which of the following cannot result in the formation of NO ?
View SolutionWhich of the following reactions will not result in the formation of anisole?
View SolutionWhich reaction results in the formation of a pair of enantiomers ?
View SolutionWhich one of the following not result in the formation of anisole?
View SolutionIn the elimination of reactions, that is, in the formation of alkenes, the reactivity of halogens in alkyl halides in the the order-
View Solution

