Number of moles of hydrogen will required for complete hydrogenation of one mole of following compound
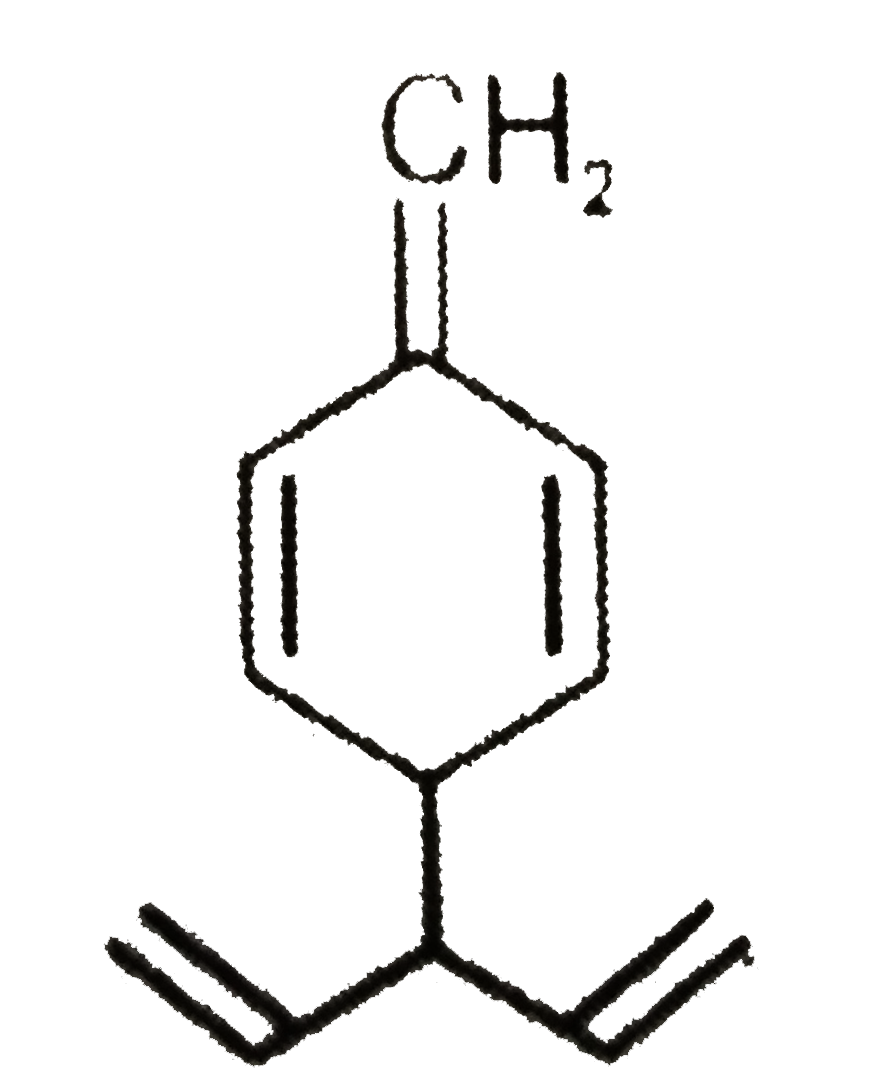
A
6
B
7
C
5
D
3
Video Solution
Text Solution
The correct Answer is:C
|
Answer
Step by step video, text & image solution for Number of moles of hydrogen will required for complete hydrogenation of one mole of following compound by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Knowledge Check
Number of moles of oxygen required for the complete combustion of butane are
A6B7.5C6.5D7The hydrogen atoms connected in the form of −OH bonds with central atom in an oxyacid is called acidic hydrogen, Oxyacid contains at least one acidic hydrogen.
Number of moles of NaOH required for the complete neutralisation of one mole of acid is maximum for :AH2S2O7BH3PO2CH3PO4DH4P2O5Number of HlO4 molecular required to complete oxidation one mole of glucose is
A4B5C6Dnone
How many moles of NaOH would be required for complete neutralization of following compounds ?

View SolutionThe minimum number of moles of O2 required for complete combustion of 1 mole of propane and 2 moles of butane is _______.
View SolutionNumber of moles of NaOH required for complete neutralization of H+ in solution which is formed by complete hydrolysis of 1 mole of PCl5
View SolutionThe number of moles of hydrogen molecules required to produce 20 moles of ammonia through Haber's process is :
View Solution1 mole of hydrogen atoms contains
View Solution

