(A) C7H14 an organic compound decolourises Br2 solution to produce (B) which combines with strong base NaNH2 to produce (C) which does not give white ppt. with AgNO3/NH4OH. (A) again when treated with Hg(OAc)2/H2O+NaBH4 to produce (D) and when treated with HBr/ROOR then OH^(-) to produce (E). Here (D) and (E) are isomeric alcohol, one is optically acetic and other is optically inactive.
Q. What is (C) ?
C−C−C−C∣∣C−Br−C−C
C−C−C−C∣C−C≡C
C−C−C|∣|CC∣−C−C
C−C−C∣∣C−Br∣C−C−C
The correct Answer is:D
Topper's Solved these Questions
- GENERAL ORGANIC CHEMISTRYBOOK - FIITJEECHAPTER - GENERAL ORGANIC CHEMISTRYEXERCISE - REASONING TYPE QUESTIONS
9 Videos
- GENERAL ORGANIC CHEMISTRYBOOK - FIITJEECHAPTER - GENERAL ORGANIC CHEMISTRYEXERCISE - MATCH THE FOLLOWING QUESTION
16 Videos
- GENERAL ORGANIC CHEMISTRYBOOK - FIITJEECHAPTER - GENERAL ORGANIC CHEMISTRYEXERCISE - MORE THAN ONE CORRECT CHOICES
24 Videos
- GASEOUS STATEBOOK - FIITJEECHAPTER - GASEOUS STATEEXERCISE - MATCHING LIST TYPE QUESTION
1 Video
- HYDROCARBONSBOOK - FIITJEECHAPTER - HYDROCARBONSEXERCISE - SINGLE INTEGER ANSWER TYPE QUESTION
9 Videos
Similar Questions
Statement-1: Formaldehyde is more reactive than benzaldehyde for nucleophilic addition reaction.
Statement-2: Formaldehyde is more stable than benzaldehyde.
View SolutionMatch the following questions:

View SolutionIn CHCl2+OH−→HCOO−, the intermediate (s) is/are
View SolutionMatch the following:
Column-IColumn-II(P) R.M.S(1)23NKT(Q) PV(2)KT(R) Average K.E.(3)√3PVM(S) Most probable speed(4)√2RTM
View SolutionDegree of unsturation in C7H8 is two. The value of y is….........
View SolutionAn organic compound (A) (C_(5)H_(7)OCl) reacts rapidly with ethanol to give (B) (C_(7)H_(12)O_(2)) . (A) also reacts with water to produce an acid which reacts with bromine to give (C ) (C_(5)H_(8)Br_(2)O_(2)) . (B) on boiling with H_(2)SO_(4) forms an acid (D). When (D) is oxidised with KMnO_(4) an acid (E ) (C_(4)H_(6)O_(3)) is produced. On mild heating, (E ) gives (F) (C_(3)H_(6)O) which cannot be oxidised by ammoniacal AgNO_(3) . Identify the compounds (A) to (F).
View SolutionWhich of the following alkenes will give optically active alcohol when treated with H_(2)O //H_(2)SO_(4)
View SolutionAn prgamoc cp,[pimd C5H9Br(A) which readily decolourises bromine water and cold alkaline KMnO4 solution gives C5H11Br(B) on catalytic hydrogenation. The reaction of A with alcoholic KOH first and then with NaNH2 produces C with evolution of NH3. C reacts with Lindlar's catalyst to give D and on reaction with Na in liquid NH3 produces E. D and E are isomers. The compound A is
View SolutionA 3^(@) optically active alcohol C_(9)H_(18)O 'A' on dehydration with conc. H_(2)SO_(4) produces B(C_(9)H_(16)) which exists in two stereoisomeric forms. For ozonolysis of B followed by work up with Zn-H_(2)O produces CH_(3)-underset(O)underset(||)(C)-H and C(C_(7)H_(12)O . C on treatment with LiAlH_(4) produces D(C_(7)H_(14)O) . D on hehydration produced Identify the correct the correct answers.
View SolutionA colourless solide (A) on hydroglysis produces a white ppt. (B) of heavy metal Solid (A) gives a clear solution in concentration HCl, however, when added to large amount of water, it again gives precipitate of (B), when H_(2)S in passed through a suspension of (A) and (B), a brown black ppt of (C ) is obtained. Compound (A) liberates a gas (D) on treating with H_(2)SO_(4) . The gas (D) is water soluble and gives white ppt. (E) with solution of mercurous salt but not with mercuric salt. The ppt white ppt (B) is of
View SolutionA colourless solide (A) on hydroglysis produces a white ppt. (B) of heavy metal Solid (A) gives a clear solution in concentration HCl, however, when added to large amount of water, it again gives precipitate of (B), when H_(2)S in passed through a suspension of (A) and (B), a brown black ppt of (C ) is obtained. Compound (A) liberates a gas (D) on treating with H_(2)SO_(4) . The gas (D) is water soluble and gives white ppt. (E) with solution of mercurous salt but not with mercuric salt. White ppt (E) is of
View SolutionAqueous ZnSO4 treated with Na2CO3/H2O produces A which when heated forms B which further treated with cobalt nitrate and warmed C is formed. A,B and C are respectively.
View Solution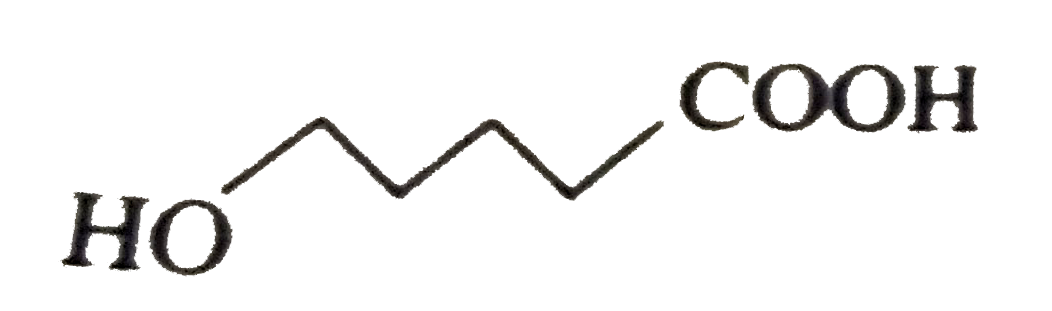
when treated with conc. H2SO4 and heated produces A. A when treated with base ΘOH produces B and consequent heating produces C:
View SolutionCompound A having molecular formula C7H7NO2 can undergo reduction with Sn+HCl treatement of A with KMnO4 gives a compound B (C7H5NO4) which has lower boiling point compared to its other isomer. B when treated with SOCl2 produces C. C when treated with CH2N2 produces D of molecular formula C8H5N3O3 D when treated with Ag/H2O produces E.
Find out structure of A :
View SolutionCompound A having molecular formula C7H7NO2 can undergo reduction with Sn+HCl treatement of A with KMnO4 gives a compound B (C7H5NO4) which has lower boiling point compared to its other isomer. B when treated with SOCl2 produces C. C when treated with CH2N2 produces D of molecular formula C8H5N3O3 D when treated with Ag/H2O produces E.
Find out structure of C :
View Solution

