Statement-1: Chloroacetaldehyde is more reactive than acetaldehyde for nucleophilic addition reaction.
Statement-2: I group in carbonyl compound makes carbonyl carbon more electron deficient.
Statement-1 is True, Statement-2 is True, Statement-2 is a correct explanation for statement-2
statement-1 is true, statement-2 is true, statement-2 is NOT a correct explanation for statement-2
statement-1 is true, statement-2 is false
statement-1 is false, statement-2 is true
The correct Answer is:A
Topper's Solved these Questions
- GENERAL ORGANIC CHEMISTRYBOOK - FIITJEECHAPTER - GENERAL ORGANIC CHEMISTRYEXERCISE - MATCH THE FOLLOWING QUESTION
16 Videos
- GENERAL ORGANIC CHEMISTRYBOOK - FIITJEECHAPTER - GENERAL ORGANIC CHEMISTRYEXERCISE - NUMERICAL BASED QUESTIONS
12 Videos
- GENERAL ORGANIC CHEMISTRYBOOK - FIITJEECHAPTER - GENERAL ORGANIC CHEMISTRYEXERCISE - COMPREHENSION TYPE
18 Videos
- GASEOUS STATEBOOK - FIITJEECHAPTER - GASEOUS STATEEXERCISE - MATCHING LIST TYPE QUESTION
1 Video
- HYDROCARBONSBOOK - FIITJEECHAPTER - HYDROCARBONSEXERCISE - SINGLE INTEGER ANSWER TYPE QUESTION
9 Videos
Similar Questions
Match the following questions:

View SolutionWhen C2H5COONa is electrolysed then what are the products obtained. Write the mol. Wt. and the corresponding subscripts of carbon hydrogen compounds.
View SolutionThe carbon-carbon double bond the carbon-carbon double bond in a molecule is capable of decolorizing Br2/CCl4 following free radical mechanism. The decolorization of Br2/water solution however shows electrophilic addition. The addition of bromine to cyclopentene provides evidence for bromonium ion immediate in bromine addition. it follows ionic mechanism. when cyclopentene reacts with bromine in presence of CCl4 anti addition occurs and gives trans-isomer so addition of halogen to an alkene is a stereospecific reaction. Cis-alkene on addition with halogen gives meso products and trans alkene on addition gives racemic mixture.
Q Trans-2-butene on reacting with Br2 in CCl4 forms mainly
View SolutionMatch the following:
Column-IColumn-II(P) R.M.S(1)23NKT(Q) PV(2)KT(R) Average K.E.(3)√3PVM(S) Most probable speed(4)√2RTM
View SolutionDegree of unsturation in C7H8 is two. The value of y is….........
View SolutionWhich carbonyl group of the given compound is most reactive for nucleophilic addition reaction ?
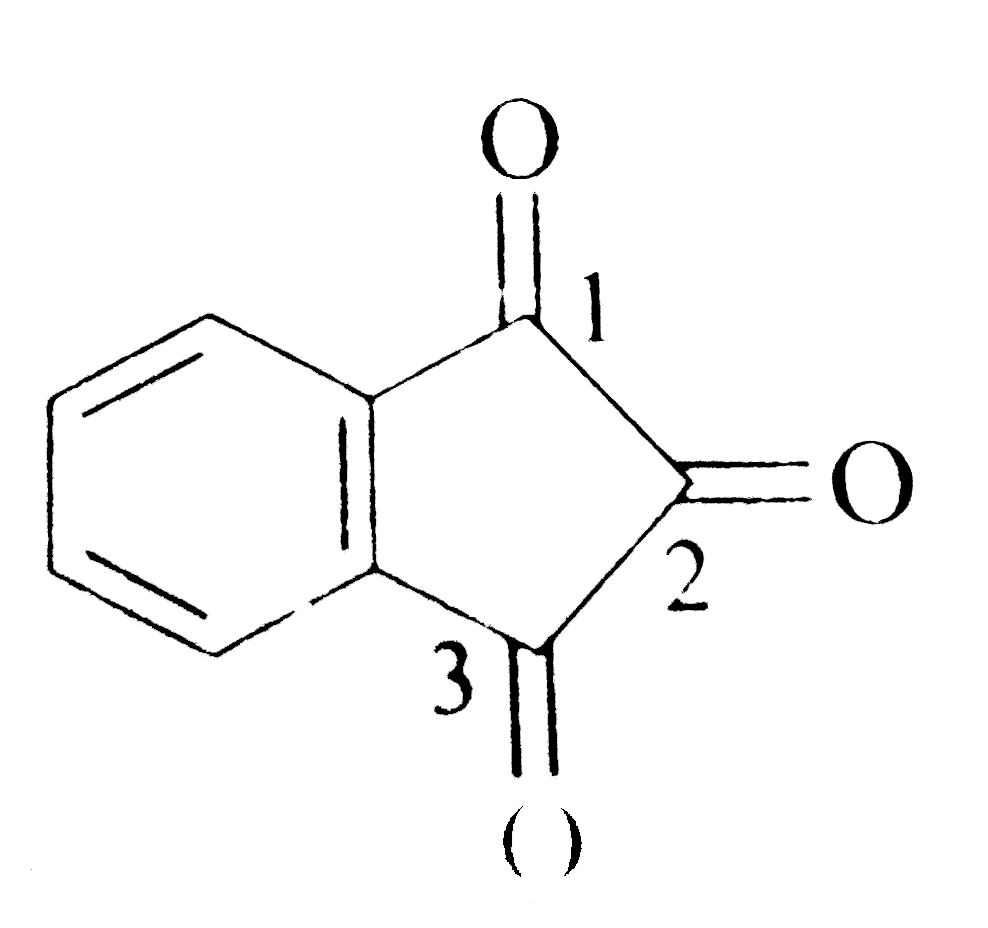
View SolutionAldehyde and ketones are specially susceptible susceptible to nucleophile addition because carbonyl group (due to electronegatvity different between carbon and oxygen). Positive charge on carbon makes it reactive towards the nucleophile. This addition is catalysed by acid. Reactivity of carbonyl compound towards nucleophilic addition increases in the electron deficience at carbonyl carbon. Thus, (-I.E.) group increase while (+I.E.) groups decrease the reactivity of carbonyl compound Which of the following is most reactve to give nucleophilic addition?
View SolutionAldehyde and ketones are specially susceptible susceptible to nucleophile addition because carbonyl group (due to electronegatvity different between carbon and oxygen). Positive charge on carbon makes it reactive towards the nucleophile. This addition is catalysed by acid. Reactivity of carbonyl compound towards nucleophilic addition increases in the electron deficience at carbonyl carbon. Thus, (-I.E.) group increase while (+I.E.) groups decrease the reactivity of carbonyl compound Carbonyl compounds show nucleophilic addition with:
View SolutionAldehyde and ketones are specially susceptible susceptible to nucleophile addition because carbonyl group (due to electronegatvity different between carbon and oxygen). Positive charge on carbon makes it reactive towards the nucleophile. This addition is catalysed by acid. Reactivity of carbonyl compound towards nucleophilic addition increases in the electron deficience at carbonyl carbon. Thus, (-I.E.) group increase while (+I.E.) groups decrease the reactivity of carbonyl compound Which among the following carbonyl compounds is most polar?
View SolutionAldehyde and ketones are specially susceptible susceptible to nucleophile addition because carbonyl group (due to electronegatvity different between carbon and oxygen). Positive charge on carbon makes it reactive towards the nucleophile. This addition is catalysed by acid. Reactivity of carbonyl compound towards nucleophilic addition increases in the electron deficience at carbonyl carbon. Thus, (-I.E.) group increase while (+I.E.) groups decrease the reactivity of carbonyl compound Select the least reactive carbonyl compound for nucleophilic addition:
View Solution(A) Carbonyl compounds take part in nucleophilic addition reactions.
(R) These reactions are initialed by nucleophilic attack at the electron deficient carbon atom.
View SolutionAldehydes and ketones ar specially susecptible to nucleophilic addition because carbonyl group is polar (due to electronegativity difference between carbon and oxygen) Positive charge on carbon makes it reactive towards the nucleophili. This addition is catalysed by acid. Reactivity of carbonyl compound towards nucleophilic addition increases with increases in the electron deficiency at carbonyl carbon. Thus, (-I.E.)groups increase while (+I.E.) groups decrease the reactivity of carbonyl compound. Which among the following is most reactive to give nucleophilic addtion ?
View SolutionAldehydes and ketones ar specially susecptible to nucleophilic addition because carbonyl group is polar (due to electronegativity difference between carbon and oxygen) Positive charge on carbon makes it reactive towards the nucleophili. This addition is catalysed by acid. Reactivity of carbonyl compound towards nucleophilic addition increases with increases in the electron deficiency at carbonyl carbon. Thus, (-I.E.)groups increase while (+I.E.) groups decrease the reactivity of carbonyl compound. Carbonyl compounds show nucleophilic addition with :
View SolutionAldehydes and ketones ar specially susecptible to nucleophilic addition because carbonyl group is polar (due to electronegativity difference between carbon and oxygen) Positive charge on carbon makes it reactive towards the nucleophili. This addition is catalysed by acid. Reactivity of carbonyl compound towards nucleophilic addition increases with increases in the electron deficiency at carbonyl carbon. Thus, (-I.E.)groups increase while (+I.E.) groups decrease the reactivity of carbonyl compound. Which among teh following carbonyl compounds is most pola?
View SolutionAldehydes and ketones ar specially susecptible to nucleophilic addition because carbonyl group is polar (due to electronegativity difference between carbon and oxygen) Positive charge on carbon makes it reactive towards the nucleophili. This addition is catalysed by acid. Reactivity of carbonyl compound towards nucleophilic addition increases with increases in the electron deficiency at carbonyl carbon. Thus, (-I.E.)groups increase while (+I.E.) groups decrease the reactivity of carbonyl compound. Select the least reactive carbonyl compound for nucleophilic addition :
View Solution

