Why boron does not form B3+ ion?
Boron has three electrons in the valence shell, because of its small size and high sum of the first three ionization enthalpies (.e.IE1+IE2+1E3) , boron does not lose all its valence electrons to form B3+ ions.
Topper's Solved these Questions
- PREPARATION AND PROPERTIES OF COMPOUNDS AND GENERAL DISCUSSION OF S-AND P-BLOCK ELEMENTSBOOK - FIITJEECHAPTER - PREPARATION AND PROPERTIES OF COMPOUNDS AND GENERAL DISCUSSION OF S-AND P-BLOCK ELEMENTSEXERCISE - SOLVED PROBLEMS (SUBJECTIVE)Level-I
29 Videos
- PREPARATION AND PROPERTIES OF COMPOUNDS AND GENERAL DISCUSSION OF S-AND P-BLOCK ELEMENTSBOOK - FIITJEECHAPTER - PREPARATION AND PROPERTIES OF COMPOUNDS AND GENERAL DISCUSSION OF S-AND P-BLOCK ELEMENTSEXERCISE - SOLVED PROBLEMS (OBJECTIVE)
27 Videos
- PERIODIC PROTERTIES OF ELEMENTSBOOK - FIITJEECHAPTER - PERIODIC PROTERTIES OF ELEMENTSEXERCISE - COMPREHENSION
13 Videos
- QUALITATIVE ANALYSIS BOOK - FIITJEECHAPTER - QUALITATIVE ANALYSIS EXERCISE - Single interger answer type
3 Videos
Similar Questions
Comment on each of the following observations:
(a) When an aqueous solution of potassium cyanide is added to aluminium sulphate Al(OH)2 is precipitated.
(b) The carbide MgaCs liberated propyne (CH3C=CH) on treatment with water.
(c) Magnesium silicate reacts with ammonium bromide in liquid ammonia to form silane,
(d)is hydrolysed by aqueous alkali at the same rate as the corresponding Si-D compound.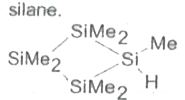
View SolutionWhich of the following statements is incorrect?
View SolutionBohr's periodic table has seven horizontal rows called periods and eighteen vertical columns called groups. Alternatively, the elements of the Bohr's table are divided into four blocks namely S- and f-blocks. Group, period and block of given element can be predicted with the help of its electronic configuration.
(I) The principle quantum number of valence shell refers to period of elements
(II) The nature of subshell (S. p or d) having last electron refers the block to which the element belongs.
(III) The number of electrons present in outermost or penultimate shell predicts the group of element.
For s-block, the group number = No. of valence electron
For p-block, the group number =10+ numbers of valence electron
For d-block, the group number =2+(n−1)d electrons
The element of atomic number 36 belongs to
View SolutionNumber of oxygen atoms in molybdate ion is
View SolutionBoron does not form B3+ ion, due to.
View SolutionAssertion:- Boron does not form N+3 ion.
Reason:- Boron does not form compound in its ground state.
View SolutionBoron does not form B^(3+) ions because
View SolutionWhy does not boron form B^(3+) ions ?
View SolutionWhy boron does not form B^(3+) ion?
View SolutionWhy is boron unable to form B^(3+) ion?
View SolutionExplain why boron connot form B3+ ions.
View SolutionExplain why boron does not form B_6^(3-) ion.
View SolutionBoron does not form B3+ ions because
View SolutionBoron does not form B^(3+) ion. Why?
View SolutionBoron does not form B3+ ion. Why?
View Solution

