Read the paragraph carefully and answer the following questions:
The property of hydrides of p-block elements mostly depends on
(i) (electronegativity difference between central atom and hydrogen..
(ii) size of central atom and
(iii)number of valence electrons in central atom. Some undergo hydrolysis in which central atom is less electronegative, Teal with OH to give hydrogen. While acidic property of hydride in a period depends on electronegativity of central atoms, I.e. more electronegative is the atom, more acidic is hydride. In a group, acidic property is proportional to the size of central atom. Some electron deficient hydride behaves as Lewis acid while only one hydride of an element in pblock behaves as Lewis base with lone pair of electrons Hydrides in which central atom's electronegativity is close to hydrogen has no reaction with water.
QWhich one is the weakest acid among the following?
HE
HCI
HBO
HI
The correct Answer is:A
Topper's Solved these Questions
- PREPARATION AND PROPERTIES OF COMPOUNDS AND GENERAL DISCUSSION OF S-AND P-BLOCK ELEMENTSBook:FIITJEEChapter:PREPARATION AND PROPERTIES OF COMPOUNDS AND GENERAL DISCUSSION OF S-AND P-BLOCK ELEMENTSExercise:MATCHING LIST TYPE QUESTIONS
Explore 2 Videos
- PREPARATION AND PROPERTIES OF COMPOUNDS AND GENERAL DISCUSSION OF S-AND P-BLOCK ELEMENTSBook:FIITJEEChapter:PREPARATION AND PROPERTIES OF COMPOUNDS AND GENERAL DISCUSSION OF S-AND P-BLOCK ELEMENTSExercise: SINGLE INTEGER ANSWER TYPE QUESTIONS
Explore 8 Videos
- PREPARATION AND PROPERTIES OF COMPOUNDS AND GENERAL DISCUSSION OF S-AND P-BLOCK ELEMENTSBook:FIITJEEChapter:PREPARATION AND PROPERTIES OF COMPOUNDS AND GENERAL DISCUSSION OF S-AND P-BLOCK ELEMENTSExercise:COMPREHENSION-VI
Explore 5 Videos
- PERIODIC PROTERTIES OF ELEMENTSBook:FIITJEEChapter:PERIODIC PROTERTIES OF ELEMENTSExercise:COMPREHENSION
Explore 13 Videos
- QUALITATIVE ANALYSIS Book:FIITJEEChapter:QUALITATIVE ANALYSIS Exercise:Single interger answer type
Explore 3 Videos
Similar Questions
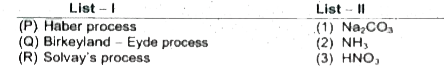
Match list - 1 with ist -- Il and select the correct answer using the codes given below.
View SolutionA 1.10g sample of copper ore is dissolved and the Cu2+ of is treated with excess KI. The liberated I2 requires 12.12mL of 0.10MNa2S2O3 solution for titration. Find the % copper by mass in ore.
View SolutionRead the paragraph carefully and answer the following questions:
Silicon is the second most abundant element occurring in earth crust. It is always found in combined state. It is obtained by reduction of silica and exists in two allotropic forms, i.e. amorphous and crystalline. Amorphous form is obtained by heating dry powdered silica with magnesium. Crystalline form is obtained by heating sand or quartz in a furnace. Silicon is used in manufacturing of certain alloys and polymers such as ferrosilicon, silicon bronze etc.
The compounds of silicon are carborundum, silicone, silicates, glass etc. Silicones are organosilicon polymer and silicates are metal derivatives of silicic acid. While carborundum are obtained by heating mixture of sand, carbon and common salt.
QWhich type of linkage is present in silicones?
View SolutionBohr's periodic table has seven horizontal rows called periods and eighteen vertical columns called groups. Alternatively, the elements of the Bohr's table are divided into four blocks namely S- and f-blocks. Group, period and block of given element can be predicted with the help of its electronic configuration.
(I) The principle quantum number of valence shell refers to period of elements
(II) The nature of subshell (S. p or d) having last electron refers the block to which the element belongs.
(III) The number of electrons present in outermost or penultimate shell predicts the group of element.
For s-block, the group number = No. of valence electron
For p-block, the group number =10+ numbers of valence electron
For d-block, the group number =2+(n−1)d electrons
The element of atomic number 36 belongs to
View SolutionNumber of oxygen atoms in molybdate ion is
View SolutionWhat is the relation between acidic nature of oxoacid and electronegativity of the central atom?
View SolutionThe property of hydrides of p-block elements mostly depends on : (i) electronegativity differnece between central atom and hydrogen. (ii) size of central atom (iii) number of valence electrons in central atom Some undergo hydrolysis in which central is less electronegative, react with OH^(-) to give hydrogen. while acidic property of hydride in a period depends on electronegativity of central atoms. i.e. more electronegative is the atom, more acidic is hydirde. In a group, acidic property is proportional to size of central atom. Some electron deficient hydride behaves as Lewis acid while only one hydride of an element in p-block behaves as Lewis base with central atom's electronegativity is close to hydrogen has no reaction with water. Which one is the weakest acid among the following ?
View SolutionThe property of hydrides of p-block elements mostly depends on : (i) electronegativity differnece between central atom and hydrogen. (ii) size of central atom (iii) number of valence electrons in central atom Some undergo hydrolysis in which central is less electronegative, react with OH^(-) to give hydrogen. while acidic property of hydride in a period depends on electronegativity of central atoms. i.e. more electronegative is the atom, more acidic is hydirde. In a group, acidic property is proportional to size of central atom. Some electron deficient hydride behaves as Lewis acid while only one hydride of an element in p-block behaves as Lewis base with central atom's electronegativity is close to hydrogen has no reaction with water. Which hydride has no reaction wiht water ?
View SolutionThe property of hydrides of p-block elements mostly depends on :
(i) electronegativity differnece between central atom and hydrogen.
(ii) size of central atom
(iii) number of valence electrons in central atom
Some undergo hydrolysis in which central is less electronegative, react with OH− to give hydrogen. while acidic property of hydride in a period depends on electronegativity of central atoms. i.e. more electronegative is the atom, more acidic is hydirde. In a group, acidic property is proportional to size of central atom. Some electron deficient hydride behaves as Lewis acid while only one hydride of an element in p-block behaves as Lewis base with central atom's electronegativity is close to hydrogen has no reaction with water.
Which one is strongest base ?
View SolutionSome of the properties which depend on electronic configuration elements are - atomic radii, ionisation potential, and electronegativity etc. The values of electronegativity of atoms A and B are 1.20 and 4 respectively. The percentage ionic character of A-B bond is:
View SolutionElectronegativity is the property of an atom in a bonded molecule.
View Solutionनिम्नलिलखित परमाणुओं के बीच बनने वाले बन्धों के प्रकार को स्पष्ट कीजिए -
(a) दो परमाणु जिनकी विद्युत-ऋणात्मकता समान है ।
(b) दो परमाणु जिनकी विद्युत-ऋणात्मकता में सूक्ष्म अन्तर है ।
(c ) दो परमाणु जिनकी विद्युत-ऋणात्मकता में अधिक अन्तर है ।
View SolutionThe property of hydrides of p-block elements mostly depends on :
(i) electronegativity differnece between central atom and hydrogen.
(ii) size of central atom
(iii) number of valence electrons in central atom
Some undergo hydrolysis in which central is less electronegative, react with OH− to give hydrogen. while acidic property of hydride in a period depends on electronegativity of central atoms. i.e. more electronegative is the atom, more acidic is hydirde. In a group, acidic property is proportional to size of central atom. Some electron deficient hydride behaves as Lewis acid while only one hydride of an element in p-block behaves as Lewis base with central atom's electronegativity is close to hydrogen has no reaction with water.
Which hydride has no reaction with water ?
View SolutionThe property of hydrides of p-block elements mostly depends on :
(i) electronegativity differnece between central atom and hydrogen.
(ii) size of central atom
(iii) number of valence electrons in central atom
Some undergo hydrolysis in which central is less electronegative, react with OH− to give hydrogen. while acidic property of hydride in a period depends on electronegativity of central atoms. i.e. more electronegative is the atom, more acidic is hydirde. In a group, acidic property is proportional to size of central atom. Some electron deficient hydride behaves as Lewis acid while only one hydride of an element in p-block behaves as Lewis base with central atom's electronegativity is close to hydrogen has no reaction with water.
Which hydride has no reaction with water ?
View SolutionRead the paragraph carefully and answer the following questions:
The property of hydrides of p-block elements mostly depends on
(i) (electronegativity difference between central atom and hydrogen..
(ii) size of central atom and
(iii)number of valence electrons in central atom. Some undergo hydrolysis in which central atom is less electronegative, Teal with OH to give hydrogen. While acidic property of hydride in a period depends on electronegativity of central atoms, I.e. more electronegative is the atom, more acidic is hydride. In a group, acidic property is proportional to the size of central atom. Some electron deficient hydride behaves as Lewis acid while only one hydride of an element in pblock behaves as Lewis base with lone pair of electrons Hydrides in which central atom's electronegativity is close to hydrogen has no reaction with water.
QWhich one is highly flammable in nature?
View Solution

