Which of the following transformation least energy is required?
A
F−(g)→F(g)+e−
B
P−(g)→P(g)+e−
C
S−(g)→S(g)+e−
D
Cl−(g)→Cl(g)+e−
Text Solution
The correct Answer is:B
If we consider the oppsite process:
P(g)+e−→P−(g), ΔH2
Order of energy releases is :ΔH4>ΔH1>ΔH3>ΔH2.
so, P−(g)→P(g)+e−, Requires least energy.
|
Topper's Solved these Questions
- PERIODIC PROPERTIESBook:VK JAISWALChapter:PERIODIC PROPERTIESExercise:Level 2
Explore 102 Videos
- PERIODIC PROPERTIESBook:VK JAISWALChapter:PERIODIC PROPERTIESExercise:MATCH THE COLUMN
Explore 13 Videos
- PERIODIC PROPERTIESBook:VK JAISWALChapter:PERIODIC PROPERTIESExercise:SUBJECTIVE PROBLEMS
Explore 22 Videos
- p-BLOCK ELEMENTSBook:VK JAISWALChapter:p-BLOCK ELEMENTSExercise:SUBJECTIVE PROBLEMS
Explore 36 Videos
- QUALITATIVE INORGANIC ANALYSISBook:VK JAISWALChapter:QUALITATIVE INORGANIC ANALYSISExercise:SUBJECTIVE PROBLEMS
Explore 5 Videos
Similar Questions
Knowledge Check
Which of the following transformation are feasible?
ACH3CH2CH2CH3AlCl3,Δ−−−−−→H3C−CH3∣C∣H−CH3BCH3−CH2−CH2−CH=CH2AlCl3−−−−−−→200−300∘CCH3−CH2−CH=CH−CH3CCH3CH2CH=CH2AlCl3−−→H2O=C∣CH3−CH3D
Which of the following transformations are feasible ?
A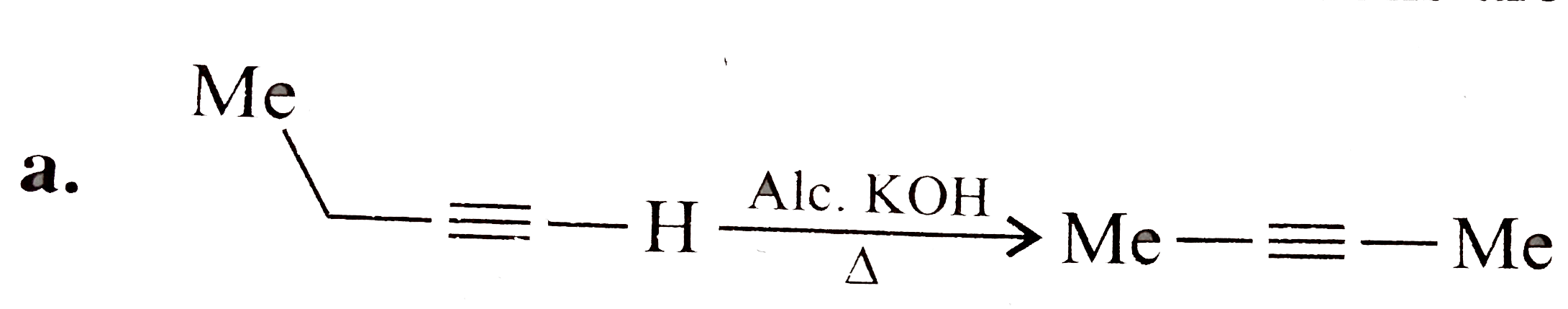 B
B C
C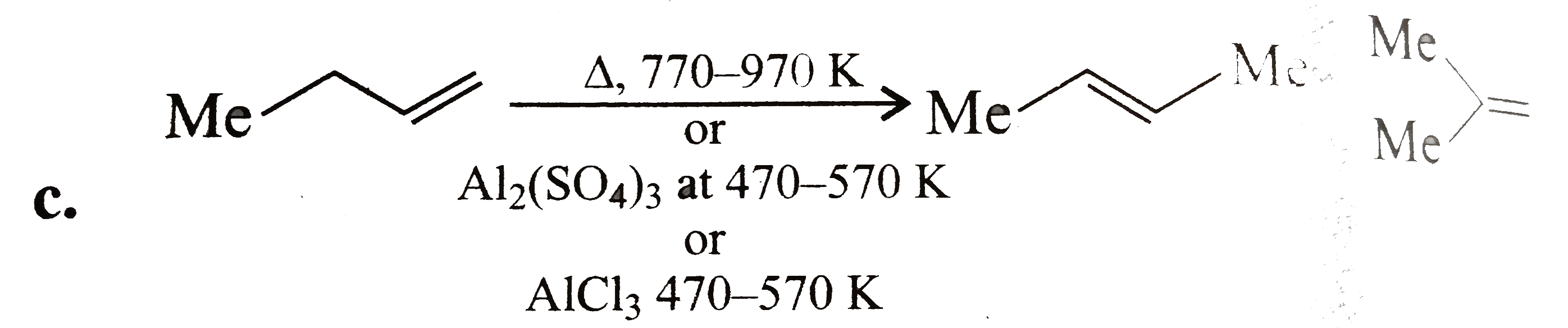 D
D
Which of the following bonds has the least energy?
ASe−SeBTe-TeCS-SDO-O
Which of the following would require least energy?
View SolutionWhich of the following shows that metabolic energy is required in the absorption of ions
View SolutionWhich of the following transformations is not correct ?
View SolutionWhich of the following transformations is not correct ?
View SolutionWhich of the following transformations is considered in defining ionization energy ?
View Solution

