Ionization energies of five elements in kcal/mol are given below:
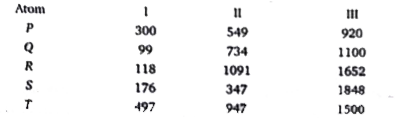
Q. Which of the following pair represents elements of same group?
A
Q,R
B
P,Q
C
P,S
D
Q,S
Text Solution
The correct Answer is:A
Both Q and R shows group in I.E2 values annd belong to alkali metals.
|
Topper's Solved these Questions
- PERIODIC PROPERTIESBook:VK JAISWALChapter:PERIODIC PROPERTIESExercise:ONE OR MORE ANSWERS IN/ARE CORRECT
Explore 99 Videos
- PERIODIC PROPERTIESBook:VK JAISWALChapter:PERIODIC PROPERTIESExercise:MATCHTHE COLUMN
Explore 5 Videos
- p-BLOCK ELEMENTSBook:VK JAISWALChapter:p-BLOCK ELEMENTSExercise:SUBJECTIVE PROBLEMS
Explore 36 Videos
- QUALITATIVE INORGANIC ANALYSISBook:VK JAISWALChapter:QUALITATIVE INORGANIC ANALYSISExercise:SUBJECTIVE PROBLEMS
Explore 5 Videos
Similar Questions
Knowledge Check
Ionization energies of five elements in kcal/mol are given below :
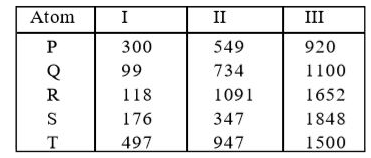
Which of the following pair represents elements of same group ?AQ, RBP, QCP, SDQ, SIonization energies of five elements in kcal/mol are given below:
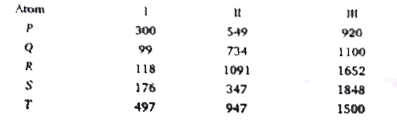
Q. Which element is a noble gas?APBTCRDSIonization energies of five elements in kcal/mol are given below :
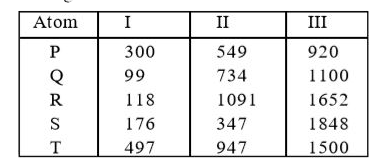
Which element is a noble gas ?APBTCRDS
Ionization energy of an element is:
View SolutionIonization energy of an element is:
View SolutionWhich of the following pair of elements have same radii?
View SolutionIonization energies of five elements in kcal/mol are given below:
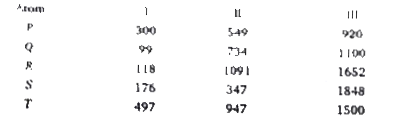
Q. Which element form stable unipositve ion?
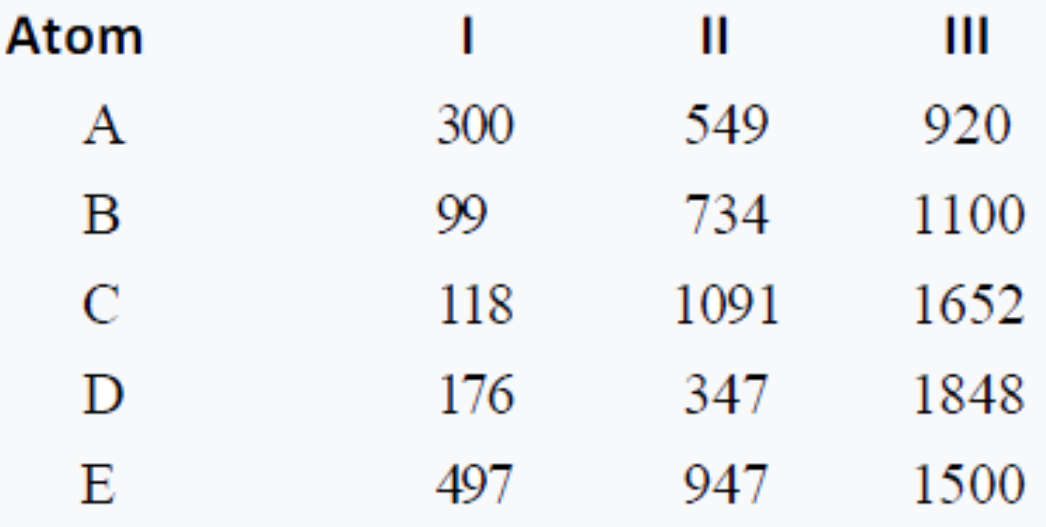
View SolutionIonization energies of five elements in kcal/mol are given below:
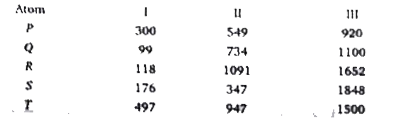
Q. Which is a non-metal (excluding noble gas)?
View Solution


 .
.