Which of the following order of molecular force of attraction among given species is incorect ?
HI>HBr>Cl2
CH3Cl>CCl4>CH4
n-pentane > iso-pentane > neo-pentane
OH2>O(CH3)2>OBr2
The correct Answer is:D
Correct order of molecular force : H2OH-bonds>OBr2Dipole-dipole∝ molecular mass>O(CH3)2
Topper's Solved these Questions
- CHEMICAL BONDING (BASIC)Book:VK JAISWALChapter:CHEMICAL BONDING (BASIC)Exercise:Level 3 (Passive 1)
Explore 6 Videos
- CHEMICAL BONDING (BASIC)Book:VK JAISWALChapter:CHEMICAL BONDING (BASIC)Exercise:Level 3 (Passive 2)
Explore 6 Videos
- CHEMICAL BONDING (ADVANCED)Book:VK JAISWALChapter:CHEMICAL BONDING (ADVANCED)Exercise:SUBJECTIVE PROBLEMS
Explore 72 Videos
- CO-ORDINATION COMPOUNDSBook:VK JAISWALChapter:CO-ORDINATION COMPOUNDSExercise:ASSERTION-REASON TYPE QUESTIONS
Explore 26 Videos
Similar Questions
According to VSEPR model, molecules adopt geometries in which their valence electron pairs position themselves as far from each other as possible. The VSEPR model considers double and triple bonds to have slightly greater repulsive effects than single bonds because of the repulsive effect of π -electrons. However the lone pair creates the maximum repulsive effect.
Which of the following statement is false ?
View SolutionAccording to VBT any covalent bond will be formed by overlapping of atomic orbitals of bonded atoms provided atomic orbitals must be half-filled and electrons be in opposite spin. According to type of overlapping covalent bonds can be classified as (a)σ-bond (b) π-bond (c ) δ-bond :
Which of the following set of orbitals does not produce nobal plane in xz-plane ?
View SolutionThere are two groups of compounds A and B. Groups A contains three compounds Px4,Qy3,Rz2. Groups B also contains three compounds Sx4,Ty3,Uz2. Hybridization of each central atom of group A compounds is same as that of iodine in IBrCl− while in group B compounds it is same as that of iodine IBrCl+. Substituents X, Y and Z exhibit covalency of one in ground state. Then find the value of x/y.
Where, x and y are total number of lone pair present at central atoms of compounds of group A and B respectively.
View SolutionAssertion: In N2 Molecule, any N-atom can coordinate with central atom/ion.
Reason: N2 molecule can also act as ambidentate ligand.
View SolutionChoose the incorect statement from among the following:
View SolutionWhich among the following has strong forces of attraction ?
View SolutionWhich among the following substances has the strongest intermolecular forces of attraction ?
View SolutionWhich among the following pairs of substances has strong intermolecular forces of attraction ?
View SolutionWhich of the following is incorrect order of increasing inter molecular attraction ?
View SolutionWhich of the following order of molecular force of attraction among given species is incorect ?
View SolutionThe type of molecular forces of attraction present in the following compounds is :
View SolutionThe intermolecular forces of attraction (i.e., H-bonding and van der waal's forces) exist among polar and non-polar species which effect melting point, boiling point, solubility and viscosity of covalent compounds : Q.The type of molecular force of attraction present in the following compounds is :
View SolutionThe type of molecular forces of attraction present in the following compounds is :
View SolutionThe intermolecular forces of attraction (i.e., H-bonding and van der waal's forces) exist among polar and non-polar species which effect melting point, boiling point, solubility and viscosity of covalent compounds :
Q.The type of molecular force of attraction present in the following compounds is :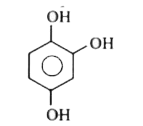
View SolutionWhich of the following have a strong inter- molecular attraction force ?
View Solution
