Hybridisation involves the mixing of orbitals having comparable energhies of same atom. Hybridised orbitals perform efficient overlapping than overlapping by pure s, p or orbitals.
Which of the following is not correctly match between given species and type of overlapping ?
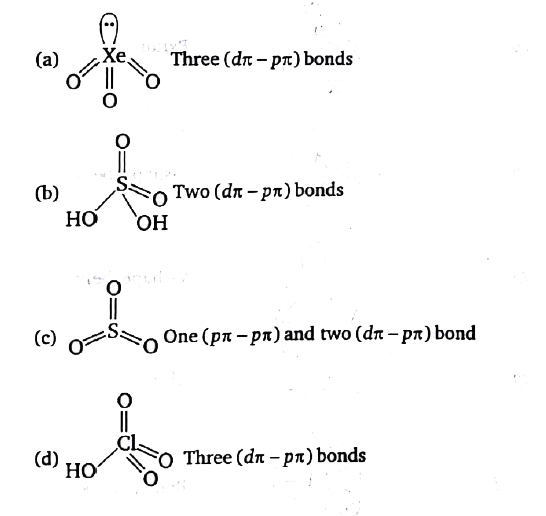
A
XeO3 : Three (dπ−pπ) bonds
B
H2SO4 : Two (dπ−pπ) bonds
C
SO3 : Three (dπ−pπ) bonds
D
HClO4 : Three (dπ−pπ) bonds
Video Solution
Text Solution
The correct Answer is:C
|
Similar Questions
Cbse | Orbital Overlap Concept | Types Of Overlapping And Nature Of Covalent Bonds | Hybridisation
View Solutionअतिसंयुग्मन निम्नलिखित में से किन कक्षकों के अतिव्यापन द्वारा होता है
View SolutionHyperconjugation involves overlap of the following orbitals :
View SolutionWhich type of the overlap of orbitals involves in hyberconjugation?
View SolutionCovalent bond is formed by the overlaping of atomic orbitals.
a) What is meant by orbital overlaping?
View SolutionCovalent bond is formed by the overlaping of atomic orbitals. What are the 3 types of overlapping ?
View Solutionएक s-कक्षक और एक p-कक्षक के अतिव्यापन के मध्य कोण है:
View SolutionWhat do you understand by overlapping of orbitals? Discuss the overlapping of p-orbitals.
View Solution