Which of the following statements is incorrect ?
Aσ - bond is weaker than aπ-bond
There are four co-ordinate bonds in the NH+4 ions
The covalent bond is directional in nature
HF is less polar than HCl
The correct Answer is:A, B, D
Topper's Solved these Questions
- CHEMICAL BONDING (BASIC)BOOK - VK JAISWALCHAPTER - CHEMICAL BONDING (BASIC)EXERCISE - MATCH THE COLUMN
25 Videos
- CHEMICAL BONDING (BASIC)BOOK - VK JAISWALCHAPTER - CHEMICAL BONDING (BASIC)EXERCISE - ASSERTION-REASON TYPE QUESTIONS
24 Videos
- CHEMICAL BONDING (BASIC)BOOK - VK JAISWALCHAPTER - CHEMICAL BONDING (BASIC)EXERCISE - Level 3 (Passive 12)
4 Videos
- CHEMICAL BONDING (ADVANCED)BOOK - VK JAISWALCHAPTER - CHEMICAL BONDING (ADVANCED)EXERCISE - SUBJECTIVE PROBLEMS
72 Videos
- CO-ORDINATION COMPOUNDSBOOK - VK JAISWALCHAPTER - CO-ORDINATION COMPOUNDSEXERCISE - ASSERTION-REASON TYPE QUESTIONS
26 Videos
Similar Questions
Column-I and Column -II contains four entries each. Entries of Column-I are to be matched with some entries of Column-II. One or more than one entries of Column-I may have the matching with the same entries of Column-II.
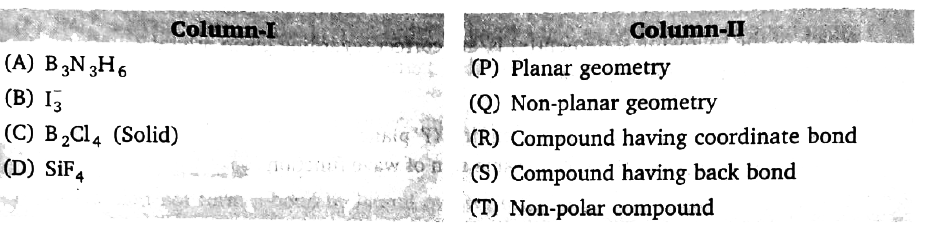
View SolutionAssertion : Multiple bond between two bonded atoms can have more than three bonds.
Reason : Multiple bond between two bonded atoms can not have more than two π-bonds.
View SolutionHybridisation involves the mixing of orbitals having comparable energhies of same atom. Hybridised orbitals perform efficient overlapping than overlapping by pure s, p or orbitals.
Which of the following is not correctly match between given species and type of overlapping ?
View SolutionThere are two groups of compounds A and B. Groups A contains three compounds Px4,Qy3,Rz2. Groups B also contains three compounds Sx4,Ty3,Uz2. Hybridization of each central atom of group A compounds is same as that of iodine in IBrCl− while in group B compounds it is same as that of iodine IBrCl+. Substituents X, Y and Z exhibit covalency of one in ground state. Then find the value of x/y.
Where, x and y are total number of lone pair present at central atoms of compounds of group A and B respectively.
View SolutionAssertion: In N2 Molecule, any N-atom can coordinate with central atom/ion.
Reason: N2 molecule can also act as ambidentate ligand.
View SolutionWhich of the following statements are incorrect
View SolutionWhich of the following statements are incorrect?
View SolutionWhich of the following is/are incorrect statements
View SolutionWhich of the following statements are incorrect?
View SolutionWhich of the following statements are incorrect?
View SolutionWhich of the following statements are incorrect ?
View SolutionWhich of the following statements are incorrect ?
View SolutionWhich of the following statements are incorrect?
View SolutionWhich are the following statements are incorrect
View SolutionWhich of the following statements is not incorrect ?
View SolutionWhich of the following statements is an incorrect statement ?
View Solution

