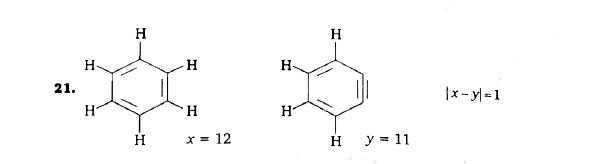
Calculate value of |x-y|, here x and y are the total number of bonds in benzene and benzyne respectively which are formed by overlapping of hybridized orbitals.
The correct Answer is:1
Similar Questions
Knowledge Check
Bond X is made by the overlap of which type of hybridized orbitals ?
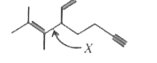 Aspandsp3Bspandsp2Csp2andsp3Dnone of these
Aspandsp3Bspandsp2Csp2andsp3Dnone of theseBond X is made by the overlep of which type of hybridized orbitals ?
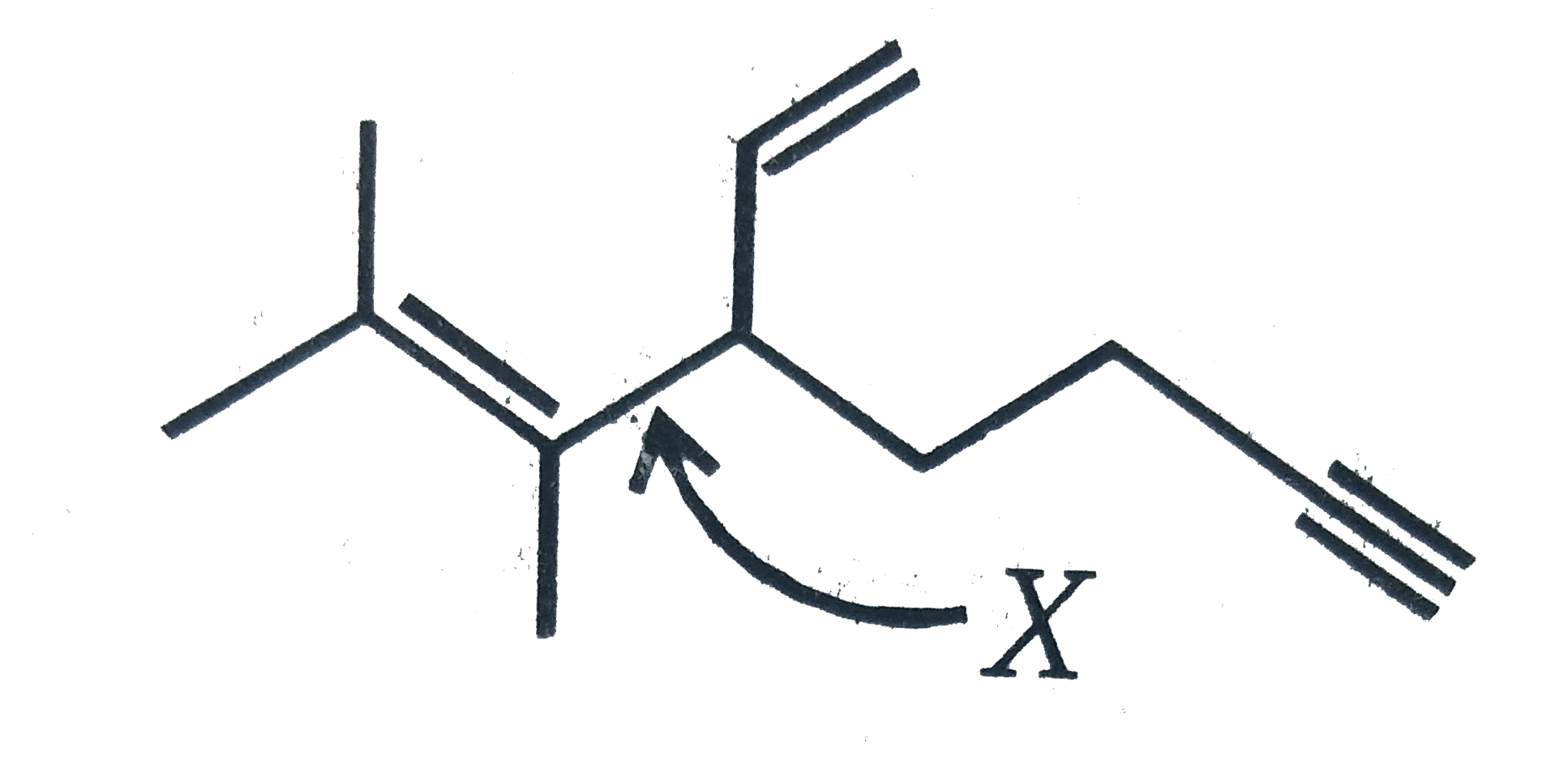 Asp and sp3Bsp and sp2Csp2andsp3Dnone of these
Asp and sp3Bsp and sp2Csp2andsp3Dnone of theseWhich of the following has a bond formed by overlap of sp3−sp, hybrid orbitals?
ACH3−C≡C−CH3BCH3−CH=CH−CH3CCH2=CH−CH=CH2DCH2=CH2
Calculate the value of " x+y-z" here x,y and z are total number of non-bonded electron pair (s),pie (π) bond(s) and sigma (σ) bonds in hydrogen phosphite ion respectively.
View SolutionCalculate the value of " x+y-z" here x,y and z are total number of non-bonded electron pair (s),pie (π) bond(s) and sigma (σ) bonds in hydrogen phosphite ion respectively.
View SolutionCalculate the value of " x+y-z" here x,y and z are total number of non-bonded electron pair (s),pie (π) bond(s) and sigma (σ) bonds in hydrogen phosphite ion respectively.
View SolutionCalculate the value of " x+y-z" here x,y and z are total number of non-bonded electron pair (s),pie (π) bond(s) and sigma (σ) bonds in hydrogen phosphite ion respectively.
View SolutionCalculate the value of " x+y-z" here x,y and z are total number of non-bonded electron pair (s),pie (π) bond(s) and sigma (σ) bonds in hydrogen phosphite ion respectively.
View Solution