Incorrect statement regarding BF3NH3 molecule is :
A
FBF bond angle <120∘
B
HNH bond angle >109∘28'
C
Exhibits intermolecular H-bond
D
hybridization of N-atom is sp3
Video Solution
Text Solution
The correct Answer is:D
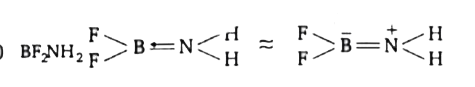
*Both boron and nitrogen are sp2-hybridised.
*FBF bond angle <120∘ (VSPER theory)
*HNH bond angle is less than 120∘ but greater than 109∘28. due to back bonding.
*Due to presence of H-atom attached to nitrogen this molecule can exhibits intermolecular H-bonding.
|
Similar Questions
The incorrect statement regarding 'X' in given reaction is: BF_(3)+LiAlH_(4) to overset("Ether")to(X)+LiF+AlF_(3)
View SolutionIncorrect statement regarding BF_(2)NH_(2) molecule is :
View SolutionClF3,BF3एवं NH3अणुओ में समतलीय त्रिकोणीय ज्यामिति वाला अणु है -
View SolutionIdentify correct statement regarding NH_(3) and BF_(3)
View SolutionSelect the correct statement(s) regarding BF2NH2 molecule:
View SolutionWhat are the shapes of (i) BF_(3) and (ii) NH_(3) molecules?
View SolutionThe incorrect statement regarding O(SiH3)2andOCl2 molecule is/are
View SolutionIncorrect statement regarding BF_(3)NH_(3) molecule is :
View Solution

