Which of the following statements are not correct?
A
All C−O bonds in CO2−3 are equal but not in H2CO3
B
All C−O bonds in HCO−2 are equal but not in HCO2H
C
C−O bond length in HCO−2 is longer than C−O bond length in CO2−3
D
C−O bond length in HCO−2andC−O bond length in CO2−3 are equal
Text Solution
The correct Answer is:C, D
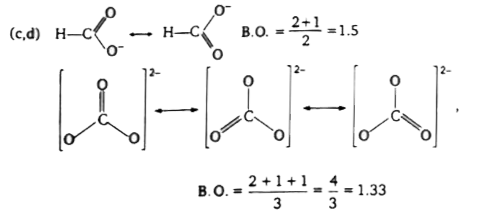
B.O=2+1+13=43=1.33
C−O bond length in HCOO− is less than C−O bond length in CO2−3
|
Topper's Solved these Questions
- CHEMICAL BONDING (ADVANCED)Book:VK JAISWALChapter:CHEMICAL BONDING (ADVANCED)Exercise:MATCH THE COLUMN
Explore 26 Videos
- CHEMICAL BONDING (ADVANCED)Book:VK JAISWALChapter:CHEMICAL BONDING (ADVANCED)Exercise:ASSERTION-REASON TYPE QUESTIONS
Explore 58 Videos
- CHEMICAL BONDING (ADVANCED)Book:VK JAISWALChapter:CHEMICAL BONDING (ADVANCED)Exercise:Level 3
Explore 92 Videos
- CHEMICAL BONDING (BASIC)Book:VK JAISWALChapter:CHEMICAL BONDING (BASIC)Exercise:Level 3 (Passive 11)
Explore 6 Videos
Similar Questions
Knowledge Check
Which of the following statements is not correct ?
AThe number of carbon atoms in an unit cell of diamond is 4BThe number of Bravais lattices in which a crystal can be categorized is 14CThe fraction of the total volume occupied by the atoms in a primitive cell is 0.48DMolecular solids are generally volatileWhich of the following statements is not correct?
APt2+ compunds are more stable than Ni2+.
Pt4+ compounds are more stable than Ni2+BPt4+ compounds are more stable than Ni2+CK2PtCl6 is well known compound but K2NiCl6 is not known.D[Pt(NH3)2Cl2] shows geometrical isomerism.Which of the following statements is not correct?
APhotographic plates are sensitive to infrared rays.BPhotographic plates are sensitive to ultraviolet rays.CInfra-red rays are invisible but can cast shadows like visible light.DInfrared photons have more energy than photons of visible light.
Which of the following statements is not correct?
View SolutionWhich of the following statements is not correct?
View SolutionWhich of the following statement is not correct ?
View SolutionWhich of the following statement is (are) correct?
View SolutionWhich of the following statements is/are correct ?
View Solution

