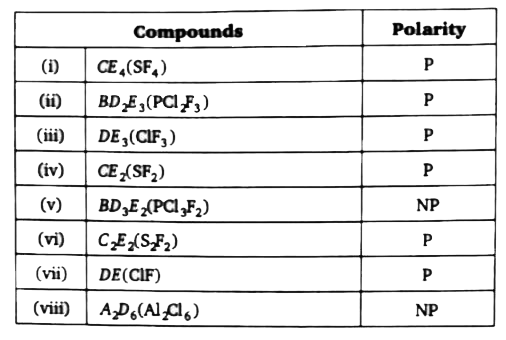
Consider the following elements A, B, C and D and their outer electronic configurtions are ns2np1,ns2np3,ns2np4andns2np5 respectively. Element E also has same outer electronic configuration like D but shows only single oxidation state(-1). If element A, B , C and D belong to same period as that of sodium. Consider the following compounds.
(i)CE4 (ii)BD2E3 (iii)DE3 (iv)DE2 (v)BD3E2 (vi)C2E2 (vii)DE (viii)A2D6.
Then calculate the value of x+y, (where x and y are total number of polar and non-polar compounds).
The correct Answer is:3
62=3, Element A=Al,B=P,C=S,D=Cl,E=F
Similar Questions
Which family of elements has the electronic configuration ns ^(2) np ^(4) ?
View SolutionAssign the position of the element having outer electronic configuration : (i) ns^(2) np ^(4) for n =3 (ii) (n-1)d ^(2) ns ^(2) for n =4 (iii) (n -2) f ^(7) (n -1) d ^(1) ns ^(2) for n =6 in the periodic table.
View SolutionAssign the position of the element having outer electronic configuration a. ns^(2)np^(4) for n=3 b. (n-1)d^(2)ns^2 for n=4 and c. (n-2) f^(7)(n-1)d^(1)ns^(2) for n=6 , in the periodic table.
View SolutionAssign the position of the element having outer electronic configuration (i) ns^(2)np^(4) " for " n = 3 (ii) (n -1) d^(2)ns^(2) " for " n = 4 and (iii) (n -2) f^(7) (n -1) d^(1) ns^(2) " for " n = 6, in the periodic table.
View SolutionThe electronic configuration of chalcogens is : 1) ns^2np^2 2) ns^2 np^3 3) ns^2 np^4 4) ns^2np^5
View SolutionAssign the position of the element having outer electronic configuration ns2np4 for n = 3, in the periodic table.
View SolutionAssign the position of the element having outer electronic configuration.
(a) ns2np4forn=3
View Solution

