Assertion: K+andNH+4 ions have many similarities in their test
Reason: Radius of K+ is almost equal to radius NH+4
If both assertion and reason are true and the reason is the correct explanation of assertion
If both assertion and reason are true and the reason is not the correct explanation of assertion
If assertion is false but reason is true
If assertion is true but the reason is false
The correct Answer is:A
Ionic radius of NH+4 is almost equal to ionic radius of K+
Topper's Solved these Questions
- s-BLOCK ELEMENTSBOOK - VK JAISWALCHAPTER - s-BLOCK ELEMENTSEXERCISE - Level 1
110 Videos
- s-BLOCK ELEMENTSBOOK - VK JAISWALCHAPTER - s-BLOCK ELEMENTSEXERCISE - MATCH THE COLUMN
9 Videos
- s-BLOCK ELEMENTSBOOK - VK JAISWALCHAPTER - s-BLOCK ELEMENTSEXERCISE - ONE OR MORE ANSWER IS/ARE CORRECT
69 Videos
- QUALITATIVE INORGANIC ANALYSISBOOK - VK JAISWALCHAPTER - QUALITATIVE INORGANIC ANALYSISEXERCISE - SUBJECTIVE PROBLEMS
5 Videos
- TYPES OF REACTIONSBOOK - VK JAISWALCHAPTER - TYPES OF REACTIONSEXERCISE - SUBJECTIVE PROBLEMS
42 Videos
Similar Questions
Sodium bicarbonate has :
View SolutionEntries of Column-I are to be matched with entrices of Column-II. Each entry of Column-I may have the matching with one or more than entries of Column-II

View SolutionThe correct statement(s) is /are
View SolutionConsider the following reaction
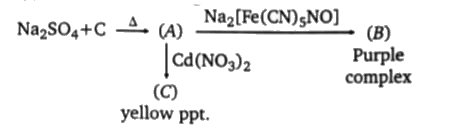
Then calculate value of ∣∣X2−Y2∣∣ (where X and Y are total number of electrons present t_(2g) and e_(g) orbitals respectively in d-block metal ion of compound B).
View SolutionFind total number of reagnets which cann produce I2 from KI solution.
Conc. H2SO4, Hg(NO3)2solution, CuSO4 solution, Conc.H3PO4,
K2Cr2O7/H+, Cl2 Water, Pb(CH3COO)2 solution, Ca(OCl)Cl/H+, NaNO2+dil.HCl
View SolutionAssertion (A): On addition of NH_(4)CI to NH_(4)OH, pH decreases but remains grater than 7 . Reason (R) : Addition of overset(o+)NH_(4) ion decreases ionication of NH_(4)OH , thus [overset(Theta)OH] decreases and also pH decreases.
View SolutionAssertion: The pH of NH_(4)OH remains unchanged on addition of NH_(4)Cl . Reason : Addition of NH_(4)Cl suppresses the dissociation of NH_(4)OH due to common ion effect.
View SolutionStatement-I : K^(+) and NH_(4)^(+) ions have lot of similarities in their test Statement-II : Radius of K^(+) is almost equal to radius of NH_(4)^(+)
View SolutionNH_(4)^(+)and Br^(-) " ions" have ionic radii of 143 pm and 196pm respectively. The coordination number of NH_(4)^(+) "ion in" NH_(4)Br is
View SolutionWhat concentration of OH^(-) ions will reduce NH_(4)^(+) ion to 2xx10^(-5)M in 0.4M solution of NH_(4)OH ? K(b)(NH_(4)OH)=1.8xx10^(-5)
View SolutionThe degree of hydrolysis of 0.05M (NH_(4))_(2)SO_(4) at 298 K is : (Given : pKa(NH_(4)^(+))=9 )
View SolutionAssertion: In the solution of K in liquid NH_(3) , blue colour appears. Reason: K reacts with NH_(3) to form KNH_(2)
View SolutionThe covalency of 'N' in NH+4 ion is equal to number of
View SolutionNH_(4)^(+) and Br^(-) ions have ionic of 143 pm and 196 pm respecitvely. The coordination number of NH_(4)^(+) ion in NH_(4)Br is
View SolutionThe NH_(4)^(+) ion is
View Solution

