The solubility of silver bromide in hypo solution is due to the formation of:
A
Ag2SO3
B
Ag2S2O3
C
[Ag(S2O3)]−
D
[Ag(S2O3)]3−
Text Solution
The correct Answer is:D
|
Topper's Solved these Questions
- d-BLOCK ELEMENTSBOOK - VK JAISWALCHAPTER - d-BLOCK ELEMENTSEXERCISE - Level 3
40 Videos
- d-BLOCK ELEMENTSBOOK - VK JAISWALCHAPTER - d-BLOCK ELEMENTSEXERCISE - ONE OR MORE ANSWER IS/ARE CORRECT
44 Videos
- d-BLOCK ELEMENTSBOOK - VK JAISWALCHAPTER - d-BLOCK ELEMENTSEXERCISE - SUBJECTIVE PROBLEMS
6 Videos
- CO-ORDINATION COMPOUNDSBOOK - VK JAISWALCHAPTER - CO-ORDINATION COMPOUNDSEXERCISE - ASSERTION-REASON TYPE QUESTIONS
26 Videos
- ENVIRONMENTAL CHEMISTRYBOOK - VK JAISWALCHAPTER - ENVIRONMENTAL CHEMISTRYEXERCISE - ASSERTION-REASON TYPE QUESTIONS
14 Videos
Similar Questions
Knowledge Check
The solubility of silver bromide in hypo solution due to the formation of
A[Ag(S2O3)]−3BAg2SO3C[Ag(S2O3)]−DAg2S2O3The solubility of silver bromide in hypo solution (excess) is due to the formation of:
AAg2SO3BAg2S2O3C[Ag(S2O3)]−D[Ag(S2O3)2]3−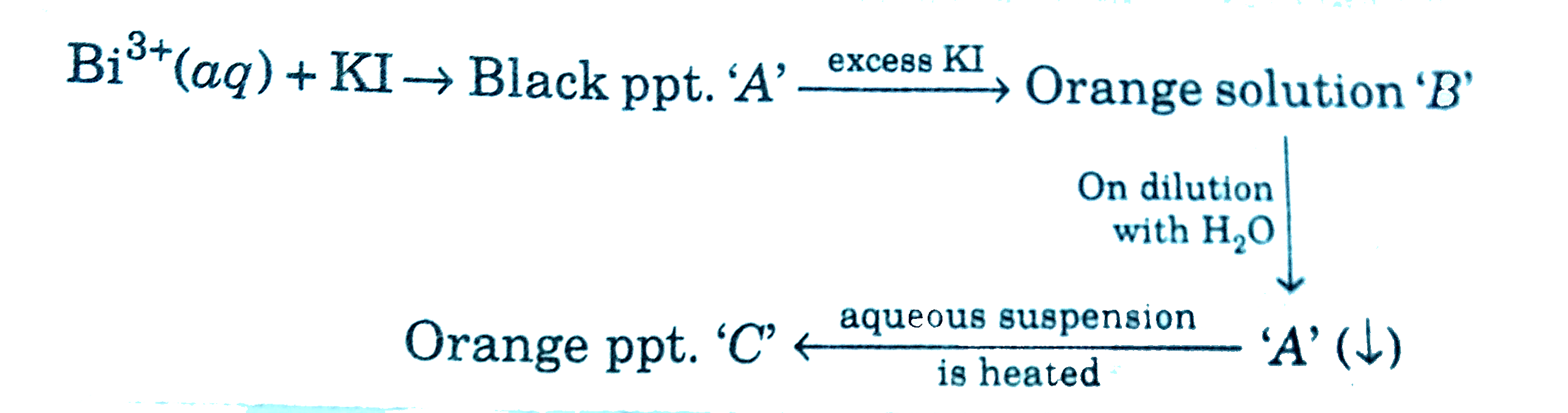
Orange solution 'B' is due to formation of:ABiI3B[BiI4]−CBi(OH)2IDBiOI
Tarnishing of silver is due to the formation of
View SolutionElectrolysis of selt solutions is due to to the formation of
View SolutionAgCl dissolves in NH3 solution, due to the formation
View SolutionSilver chloride is soluble in methylamine due to the formation of:
View SolutionSilver chloride is soluble in methylamine due to the formation of :
View Solution

