K2[Cd(CN)4]+H2S→CdS⏐⏐↓+2KCN+2HCN
For precipitate formation formation reaction
For precipitate dissolution reaction
For precipitate exchange reaction
For no reaction
The correct Answer is:a
Step by step video, text & image solution for K_(2)[Cd(CN)_(4)]+H_(2)S to CdS darr+2KCN + 2HCN by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Which of the following metal nitrate produces gaseous product when reacts with KCN solution?
View Solution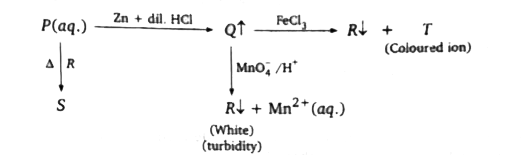
Q. Species P and S are respectively:
View Solution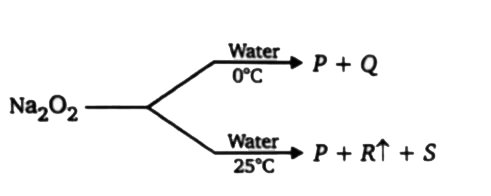
Calculate sum of bond order between same bonded atoms in Q and R compounds.
View SolutionAssertion :When H2S is passed through a solution containing [Cu(CN)4]2− and [Cd(CN)4]2− ions ,only cadinilum precipitate as CdS.
Reasion : The oxidation state and coordination number of cadmium in [Cd(CN)4]2− are 2 and 4 respectively.
View SolutionFe(CN)_(2)darr+4KCN to K_(4)[Fe(CN)_(6)]
View SolutionCuSO4+2KCN→CuCN⏐↓+(CN)2↑⏐+K2SO4
View SolutionCd(CN)2⏐⏐↓+2KCN→K2[Cd(CN)4]
View SolutionK2[Cd(CN)4]+H2S→CdS⏐⏐↓+2KCN+2HCN
View SolutionAssertion: When H2S is passed through a solution containing [Cu(CN)4]2−and[Cd(CN)4]2− ions, only cadmium precipitates as CdS.
Reason: The oxidation state and co-ordination number of cadmium in [Cd(CN)4]2− are II and 4 respectively.
View Solution[Cd(CN)4]2−में H2S प्रवाहित करने पर पीला अवक्षेप क्यों आता है?
View SolutionFe(CN)2⏐⏐↓+4KCN→K4[Fe(CN)6]
View SolutionCd(CN)2⏐⏐↓+2KCN→K2[Cd(CN)4]
View Solution4KCN+Fe(CN)2⏐⏐↓→K4[Fe(CN)6]
View SolutionFe(CN)_(2)darr+4KCN to K_(4)[Fe(CN)_(6)]
View SolutionCuSO4+2KCN→CuCN⏐↓+(CN)2↑⏐+K2SO4
View Solution

