In which of these cyclic compounds you find the minimum angle strain?
C3H6
C4H8
C5H10
C6H12
The correct Answer is:D
To determine which cyclic compound has the minimum angle strain, we need to analyze the angle strain associated with each of the given cyclic compounds: C3H6 (cyclopropane), C4H8 (cyclobutane), C5H10 (cyclopentane), and C6H12 (cyclohexane).
1. Identify the Compounds:
- C3H6 is cyclopropane.
- C4H8 is cyclobutane.
- C5H10 is cyclopentane.
- C6H12 is cyclohexane.
2. Determine the Hybridization:
- All the compounds are made up of carbon atoms that are sp³ hybridized since they are cycloalkanes. Each carbon in these compounds forms four sigma bonds and has no lone pairs.
3. Calculate the Ideal Bond Angle:
- For sp³ hybridized carbon, the ideal bond angle is approximately 109.5 degrees.
4. Analyze Each Compound:
- Cyclopropane (C3H6):
- The structure is triangular, and the bond angles are 60 degrees.
- Angle strain = Ideal angle (109.5°) - Actual angle (60°) = 49.5°.
- Cyclobutane (C4H8):
- The structure is square-like, and the bond angles are approximately 90 degrees.
- Angle strain = Ideal angle (109.5°) - Actual angle (90°) = 19.5°.
- Cyclopentane (C5H10):
- The structure is pentagonal, and the bond angles are approximately 108 degrees.
- Angle strain = Ideal angle (109.5°) - Actual angle (108°) = 1.5°.
- Cyclohexane (C6H12):
- The structure can adopt a chair conformation, which allows for bond angles close to 109.5 degrees.
- Angle strain is minimal, effectively 0° in the chair form.
5. Conclusion:
- Among the compounds analyzed, cyclohexane (C6H12) has the least angle strain due to its ability to adopt a stable chair conformation with bond angles very close to the ideal angle of 109.5 degrees.
Thus, the cyclic compound with the minimum angle strain is C6H12 (cyclohexane).
- C3H6 is cyclopropane.
- C4H8 is cyclobutane.
- C5H10 is cyclopentane.
- C6H12 is cyclohexane.
- All the compounds are made up of carbon atoms that are sp³ hybridized since they are cycloalkanes. Each carbon in these compounds forms four sigma bonds and has no lone pairs.
- For sp³ hybridized carbon, the ideal bond angle is approximately 109.5 degrees.
- Cyclopropane (C3H6):
- The structure is triangular, and the bond angles are 60 degrees.
- Angle strain = Ideal angle (109.5°) - Actual angle (60°) = 49.5°.
- Cyclobutane (C4H8):
- The structure is square-like, and the bond angles are approximately 90 degrees.
- Angle strain = Ideal angle (109.5°) - Actual angle (90°) = 19.5°.
- Cyclopentane (C5H10):
- The structure is pentagonal, and the bond angles are approximately 108 degrees.
- Angle strain = Ideal angle (109.5°) - Actual angle (108°) = 1.5°.
- Cyclohexane (C6H12):
- The structure can adopt a chair conformation, which allows for bond angles close to 109.5 degrees.
- Angle strain is minimal, effectively 0° in the chair form.
- Among the compounds analyzed, cyclohexane (C6H12) has the least angle strain due to its ability to adopt a stable chair conformation with bond angles very close to the ideal angle of 109.5 degrees.
Similar Questions
Knowledge Check
In which of the following compound Mn shoes minimum radius ?
AMnO2BKmNo4CMnODNone of theseWhich of the following has maximum angle strain ?
APropaneBCyclopropaneCn - butaneDCyclobutaneWhich of the following has maximum angle strain ?
A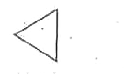 B
B C
C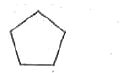 D
D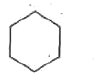
In which of the following has minimum torsional strain and minimum vander wall strain.
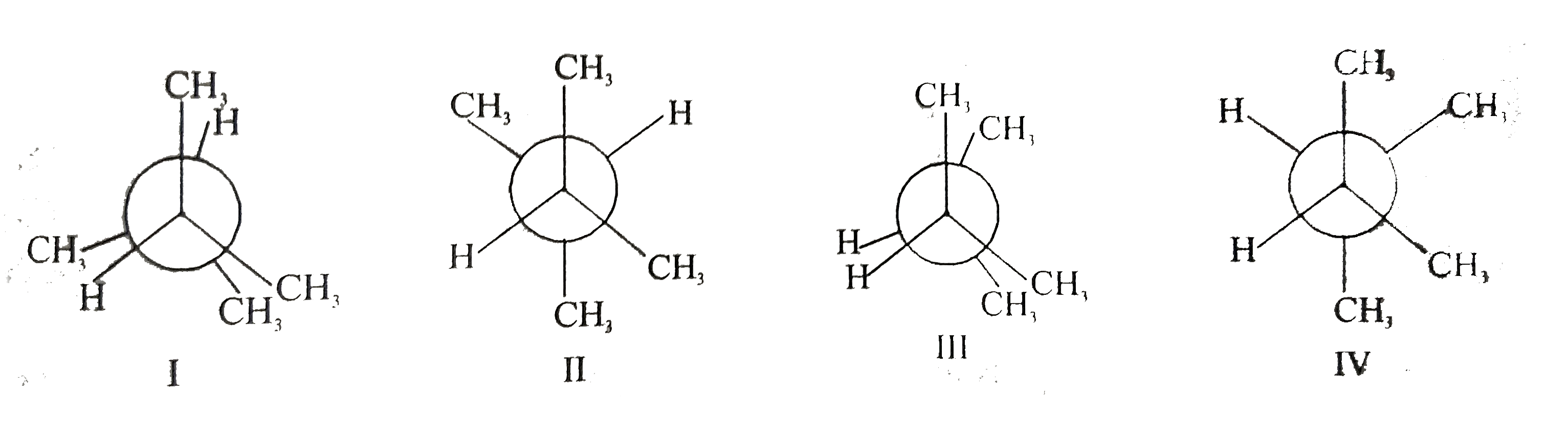
View SolutionWhich of the following is NOT a cyclic compound ?
View SolutionWhich of the following is hetero cyclic compound?
View SolutionWhich of the following is not a cyclic compound?
View SolutionWhich of the following will not yeild a cyclic compound on heating
View Solution
