Which of the following has maximum freezing point is
Pure H2O
0.1 M NaCl (aq)
0.01 M NaCl (aq)
0.5M NaCl (aq)
The correct Answer is:A
To determine which of the given substances has the maximum freezing point, we need to analyze the effects of solutes on the freezing point of a solvent, specifically water in this case.
1. Understanding Freezing Point Depression:
- When a non-volatile solute (like NaCl) is added to a pure solvent (like water), the freezing point of the solvent decreases. This phenomenon is known as freezing point depression.
2. Identifying the Substances:
- The options given are:
- Pure water
- NaCl (sodium chloride)
- We need to compare the freezing points of these substances.
3. Freezing Point of Pure Water:
- The freezing point of pure water is 0°C. This is the baseline freezing point for comparison.
4. Freezing Point of NaCl Solution:
- When NaCl is dissolved in water, it dissociates into Na⁺ and Cl⁻ ions. The presence of these ions lowers the freezing point of the solution compared to pure water.
- The more solute particles present, the greater the depression of the freezing point.
5. Conclusion:
- Since pure water has a freezing point of 0°C and the addition of NaCl lowers the freezing point of the solution, we can conclude that pure water has the maximum freezing point compared to the NaCl solution.
Final Answer:
Pure water has the maximum freezing point.
- When a non-volatile solute (like NaCl) is added to a pure solvent (like water), the freezing point of the solvent decreases. This phenomenon is known as freezing point depression.
- The options given are:
- Pure water
- NaCl (sodium chloride)
- We need to compare the freezing points of these substances.
- The freezing point of pure water is 0°C. This is the baseline freezing point for comparison.
- When NaCl is dissolved in water, it dissociates into Na⁺ and Cl⁻ ions. The presence of these ions lowers the freezing point of the solution compared to pure water.
- The more solute particles present, the greater the depression of the freezing point.
- Since pure water has a freezing point of 0°C and the addition of NaCl lowers the freezing point of the solution, we can conclude that pure water has the maximum freezing point compared to the NaCl solution.
Pure water has the maximum freezing point.
Similar Questions
Knowledge Check
Which of the following has minimum freezing point
A0.1 M K2Cr2O7B0.1 M NH4CIC0.1 M BaSO4D0.1 M Al2(SO4)3Which of the following has maximum boiling point?
A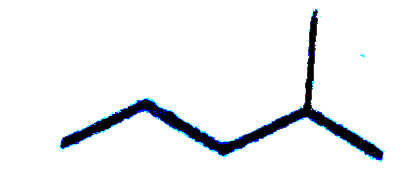 B
B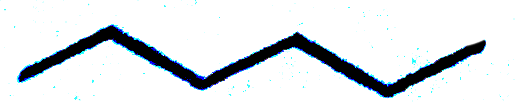 C
C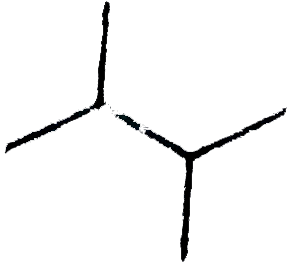 D
D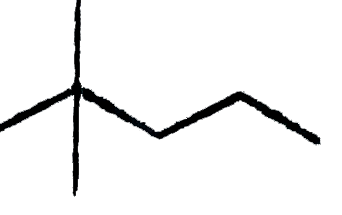
Which of the following has maximum boiling point ?
ACH3CH2CH2CHOBCH3CH2CHOCCH3CHODHCHO

