Which of the following does not show optical isomerism?
[Co(en)3]3+
trans [CoCl2(en)2]+
[Cr(⊗)3]+2
cis[PtCl2(en)2]+2
The correct Answer is:B
To determine which of the given coordination compounds does not show optical isomerism, we need to analyze the symmetry properties of each compound. Optical isomerism occurs when a compound can exist in two non-superimposable mirror images, which typically happens in chiral molecules. A compound is considered achiral (and thus does not show optical isomerism) if it possesses a plane of symmetry.
1. Identify the Coordination Compounds:
We need to examine the coordination compounds provided in the question. Let's assume we have four options, including bidentate ligands like ethylene diamine (en) and others.
2. Determine Coordination Numbers:
- For the first compound, which has ethylene diamine (en) as a bidentate ligand, the coordination number is 6, indicating an octahedral geometry.
- For the second compound, analyze if it has symmetrical ligands that can lead to a plane of symmetry.
- For the third compound, check if it has a similar structure to the first and if it can show optical isomerism.
- For the fourth compound, also check its structure and symmetry.
3. Analyze Symmetry:
- Draw the structures of the compounds.
- For the first compound (en), it is likely to be optically active due to lack of symmetry.
- For the second compound, if it has symmetrical ligands positioned opposite each other (trans configuration), it will have a plane of symmetry and thus be optically inactive.
- The third compound may also be optically active if it lacks symmetry.
- The fourth compound (cis configuration) may also lack symmetry, making it optically active.
4. Conclusion:
After analyzing the symmetry of each compound, we conclude that the second compound, which has a plane of symmetry due to its trans configuration, does not show optical isomerism. Therefore, it is the correct answer.
Final Answer:
The compound that does not show optical isomerism is the second option (the one with a trans configuration).
We need to examine the coordination compounds provided in the question. Let's assume we have four options, including bidentate ligands like ethylene diamine (en) and others.
- For the first compound, which has ethylene diamine (en) as a bidentate ligand, the coordination number is 6, indicating an octahedral geometry.
- For the second compound, analyze if it has symmetrical ligands that can lead to a plane of symmetry.
- For the third compound, check if it has a similar structure to the first and if it can show optical isomerism.
- For the fourth compound, also check its structure and symmetry.
- Draw the structures of the compounds.
- For the first compound (en), it is likely to be optically active due to lack of symmetry.
- For the second compound, if it has symmetrical ligands positioned opposite each other (trans configuration), it will have a plane of symmetry and thus be optically inactive.
- The third compound may also be optically active if it lacks symmetry.
- The fourth compound (cis configuration) may also lack symmetry, making it optically active.
After analyzing the symmetry of each compound, we conclude that the second compound, which has a plane of symmetry due to its trans configuration, does not show optical isomerism. Therefore, it is the correct answer.
The compound that does not show optical isomerism is the second option (the one with a trans configuration).
Topper's Solved these Questions
- ALCOHOLS, PHENOLS AND ETHERSBook:AAKASH INSTITUTEChapter: ALCOHOLS, PHENOLS AND ETHERSExercise:Try yourself
Explore 4 Videos
- ALCOHOLS, PHENOLS AND ETHERSBook:AAKASH INSTITUTEChapter: ALCOHOLS, PHENOLS AND ETHERSExercise:Exercise
Explore 40 Videos
- ALCOHOLS, PHENOLS AND ETHERS Book:AAKASH INSTITUTEChapter:ALCOHOLS, PHENOLS AND ETHERS Exercise:Try Yourself
Explore 5 Videos
Similar Questions
Knowledge Check
which of the following sdoes not show optical isomerism ?
A[Co(NH3)3Cl3]B[Co(en)Cl2(NH3)2]+C[Co(en)3]3+D[Co(en)2Cl2]+
(e = ethylenediammine)Which of the following will not show optical isomerism ?
ACl-CH=C=C=CH-ClBCl-CH=C=C=C=CH-ClC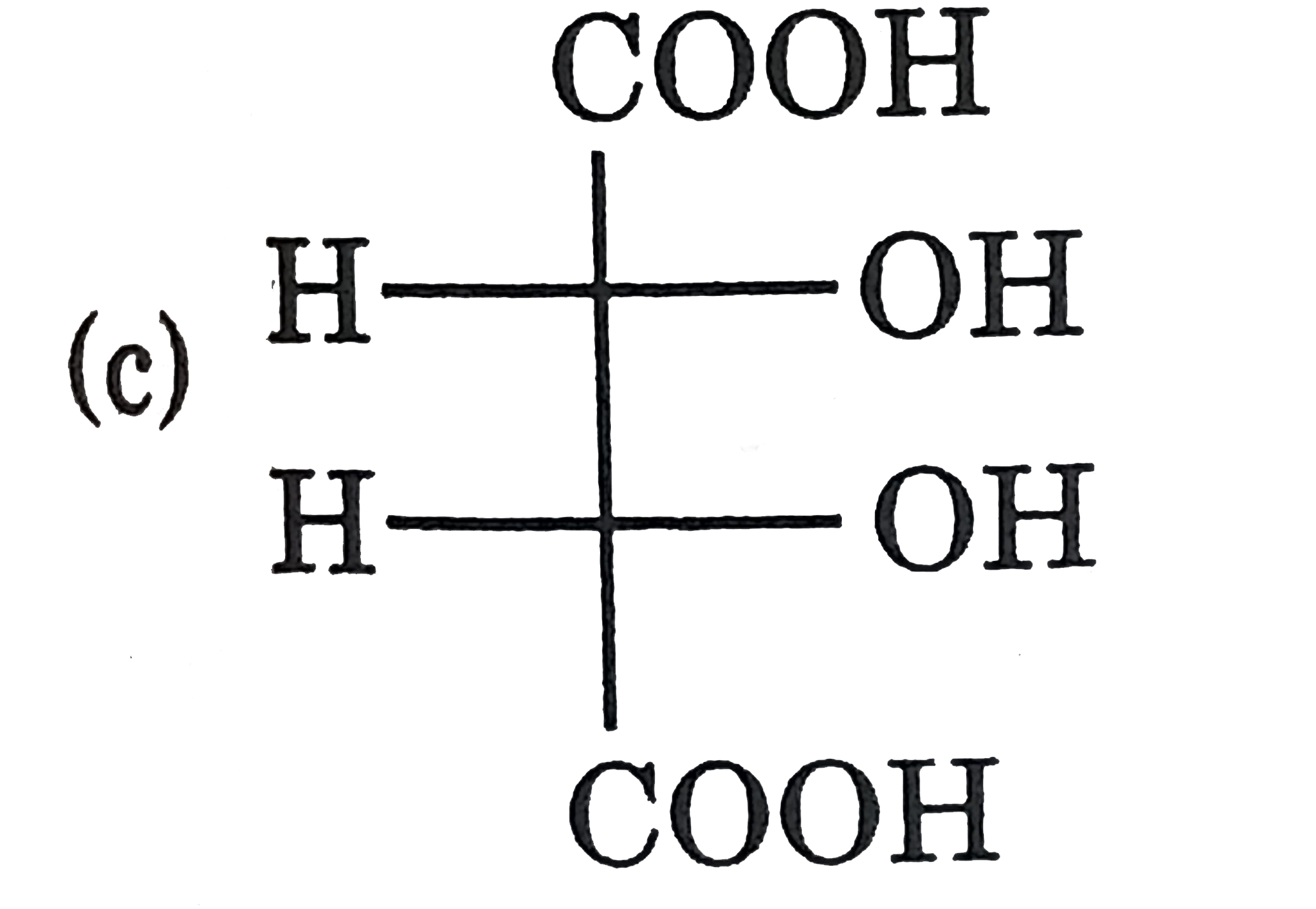 D
D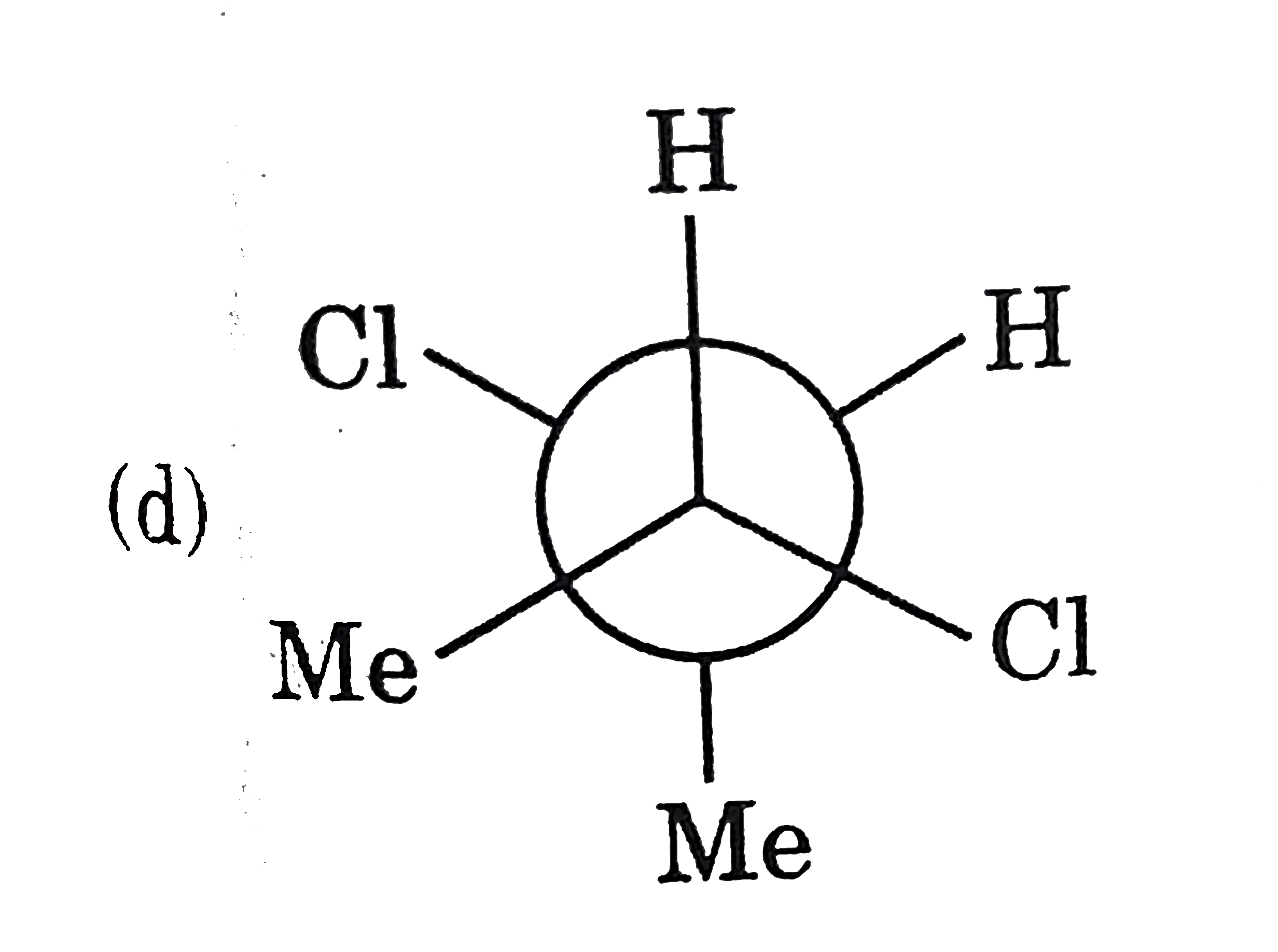
Which of the following will not show optical isomerism ?
ACl−CH=C=CH−ClBBr−CH=C=CH−BrC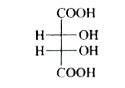 D
D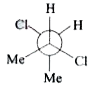
Which of the following does not show geometrical isomerism?
View SolutionWhich of the following does not show geometrical isomerism?
View SolutionWhich of the following does not show geometrical isomerism?
View SolutionWhich of the following does not show geometrical isomerism
View Solution. Which of the following does not show geometrical isomerism?
View Solution
