Which of the following is not the isomer of CH3COOH?
H−O∣∣C−O∣∣C−H
H−O∣∣C−OCH3
H2OH∣C−O∣∣C=H
All of these
To determine which of the given compounds is not an isomer of acetic acid (CH₃COOH), we need to analyze the molecular formula and the number of atoms present in each option.
1. Identify the Molecular Formula of Acetic Acid:
- Acetic acid has the molecular formula CH₃COOH, which can be simplified to C₂H₄O₂.
- This indicates that acetic acid contains 2 carbon atoms, 4 hydrogen atoms, and 2 oxygen atoms.
2. Understand the Concept of Isomers:
- Isomers are compounds that have the same molecular formula but different structural arrangements or functional groups.
- For a compound to be an isomer of acetic acid, it must also have the formula C₂H₄O₂.
3. Examine the Given Options:
- List the options provided in the question (not given in the transcript, but let's assume some common ones):
- (a) Ethanol (C₂H₅OH)
- (b) Dimethyl ether (C₂H₆O)
- (c) Acetic anhydride (C₄H₆O₃)
- (d) Propanoic acid (C₃H₆O₂)
4. Count Atoms in Each Option:
- Ethanol (C₂H₅OH): 2 Carbons, 6 Hydrogens, 1 Oxygen (Not an isomer)
- Dimethyl ether (C₂H₆O): 2 Carbons, 6 Hydrogens, 1 Oxygen (Not an isomer)
- Acetic anhydride (C₄H₆O₃): 4 Carbons, 6 Hydrogens, 3 Oxygens (Not an isomer)
- Propanoic acid (C₃H₆O₂): 3 Carbons, 6 Hydrogens, 2 Oxygens (Not an isomer)
5. Identify the Compound that is Not an Isomer:
- From the analysis, we see that Ethanol (C₂H₅OH), Dimethyl ether (C₂H₆O), Acetic anhydride (C₄H₆O₃), and Propanoic acid (C₃H₆O₂) do not have the same molecular formula as acetic acid (C₂H₄O₂).
- Therefore, any compound that does not have the same number of carbon, hydrogen, and oxygen atoms as acetic acid is not an isomer.
Conclusion:
The compound that is not an isomer of CH₃COOH is Acetic anhydride (C₄H₆O₃).
- Acetic acid has the molecular formula CH₃COOH, which can be simplified to C₂H₄O₂.
- This indicates that acetic acid contains 2 carbon atoms, 4 hydrogen atoms, and 2 oxygen atoms.
- Isomers are compounds that have the same molecular formula but different structural arrangements or functional groups.
- For a compound to be an isomer of acetic acid, it must also have the formula C₂H₄O₂.
- List the options provided in the question (not given in the transcript, but let's assume some common ones):
- (a) Ethanol (C₂H₅OH)
- (b) Dimethyl ether (C₂H₆O)
- (c) Acetic anhydride (C₄H₆O₃)
- (d) Propanoic acid (C₃H₆O₂)
- Ethanol (C₂H₅OH): 2 Carbons, 6 Hydrogens, 1 Oxygen (Not an isomer)
- Dimethyl ether (C₂H₆O): 2 Carbons, 6 Hydrogens, 1 Oxygen (Not an isomer)
- Acetic anhydride (C₄H₆O₃): 4 Carbons, 6 Hydrogens, 3 Oxygens (Not an isomer)
- Propanoic acid (C₃H₆O₂): 3 Carbons, 6 Hydrogens, 2 Oxygens (Not an isomer)
- From the analysis, we see that Ethanol (C₂H₅OH), Dimethyl ether (C₂H₆O), Acetic anhydride (C₄H₆O₃), and Propanoic acid (C₃H₆O₂) do not have the same molecular formula as acetic acid (C₂H₄O₂).
- Therefore, any compound that does not have the same number of carbon, hydrogen, and oxygen atoms as acetic acid is not an isomer.
The compound that is not an isomer of CH₃COOH is Acetic anhydride (C₄H₆O₃).
Similar Questions
Knowledge Check
Which of the following is not an isomer of CH3−CH2CHO
ACH3−CH2−O∣∣C−CH3B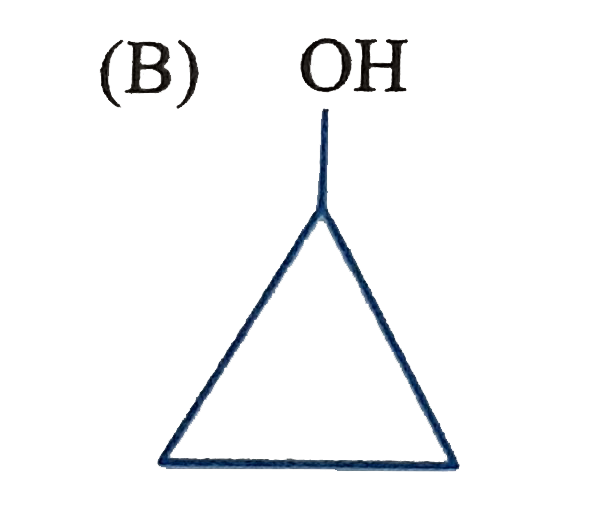 C
C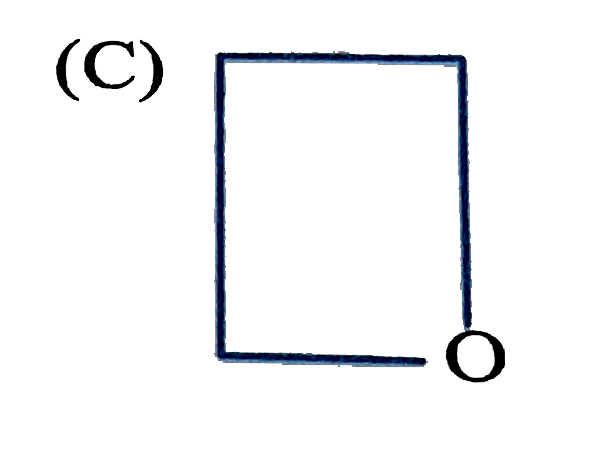 DCH2=CH−CH2−OH
DCH2=CH−CH2−OHAn aqueous solution of which of the following concentration of CH3COOH is the best conductor.
A101MB10−3MC10−1MD102MWhich one of the following structure is not an isomer of the compound CH3 - CO - CH2CH2CH2CH3?
ACH3CH2OCH=CHCH2CH3BCH3CH=CHCH2CH2CHOC(CH3)2CH−CO−CH2CH3DCH3CH2COCH2CH2CH3
which is not soluble in CH3COOH ?
View SolutionWhich of the following is a/are strong acids HNO3,HCl,CH3COOH,H2CO3
View SolutionWhich of the following is the empirical formula of acetic acid (CH3COOH)?
View SolutionWhich one of the following is the correct order of acidic strength?
(i) Cl3C−COOH
(ii) Cl2CH−COOH
(iii) ClCH2−COOH
(iv) CH3−COOH
View SolutionWhich of following aqueous mixture of CH3COOH and CH3COONa will have maximum buffer capacity?
View Solution

