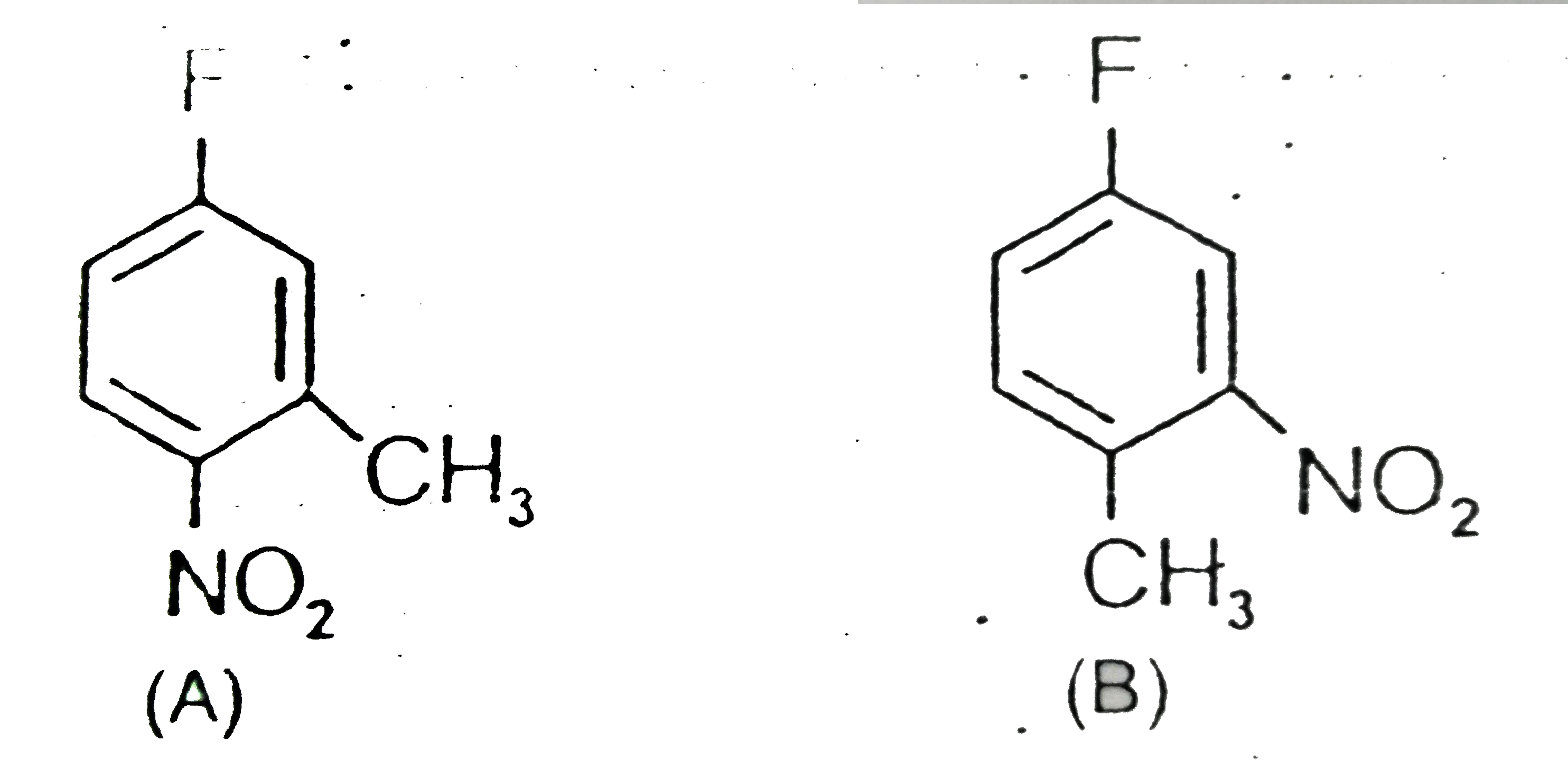
Which one of the folowing compounds readily reacts with NaOH ?
Video Solution
Text Solution
Compound ( A) will readily react with NaOH because it has a strongly electron - withdrawing group (NO2) at the para position with respect to fluorine .

Compound (B) however does not react with NaOH under normal conditions as it does not have NO2 group either at ortho or para with respect to fluorine .
|
Topper's Solved these Questions
- HALOALKANES AND HALOARENES Book:AAKASH INSTITUTEChapter:HALOALKANES AND HALOARENES Exercise:Illustration
Explore 5 Videos
- HALOALKANES AND HALOARENES Book:AAKASH INSTITUTEChapter:HALOALKANES AND HALOARENES Exercise:Assignment (Section - A ) ( Competition Level Question )
Explore 50 Videos
- GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTSBook:AAKASH INSTITUTEChapter:GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTSExercise:Try Yourself
Explore 33 Videos
- HYDROCARBONSBook:AAKASH INSTITUTEChapter:HYDROCARBONSExercise:Assignment(Section - D) (Assertion -Reason Type Question)
Explore 24 Videos
Similar Questions
Knowledge Check
Which of the following compound will react with aq. NaOH?
A B
B C
C D
D
Which of the following compounds does not react with NaOH ?
ACH3COOHBCH3CONH2CC6H5OHDCH3CH2OHWhich of the following compounds does not react with NaOH?
ACH3COOHBCH3CONH2CC6H5OHDCH3CH2OH
Which one of the following compounds react with methylamagnesium iodile?
View SolutionWhich of the following compounds are readily soluble in water ?
View SolutionWhich one of the following compounds most readily undergoes substitution by SN2 mechanism ?
View SolutionWhich of the following compounds will be most easily attacked by an electrophile?
View SolutionWhich one of the following compounds will be most readily attacked by an electrophile?
View Solution