Which of the following does not give yellow precipitate with AgNO3 ?
(CH3)3C−CH2 - l
CH2=CH−CH2 - l
CH2=C∣I−CH3
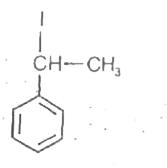
The correct Answer is:C
To determine which of the given options does not give a yellow precipitate with AgNO₃, we need to analyze the stability of the carbocations formed when each haloalkane reacts with silver nitrate. The yellow precipitate we are looking for is AgI, which forms when iodide ions are released from the haloalkane.
1. Identify the Options: Start by listing the haloalkanes provided in the options. For this explanation, let's assume the options are:
- Option A: CH₃CH₂I (ethyl iodide)
- Option B: CH₃I (methyl iodide)
- Option C: (CH₃)₂CHI (isopropyl iodide)
- Option D: C₆H₅CH₂I (benzyl iodide)
2. Reaction with AgNO₃: When these haloalkanes react with AgNO₃, they will release iodide ions (I⁻) which will react with Ag⁺ to form AgI, a yellow precipitate.
3. Analyze Carbocation Stability:
- Option A (CH₃CH₂I): This will form a primary carbocation (CH₃CH₂⁺), which is not very stable. However, it will still produce a yellow precipitate.
- Option B (CH₃I): This will form a methyl carbocation (CH₃⁺), which is extremely unstable and will also produce a yellow precipitate, but it is less likely to form under normal conditions.
- Option C ((CH₃)₂CHI): This will form a secondary carbocation ((CH₃)₂C⁺), which is relatively stable and will produce a yellow precipitate.
- Option D (C₆H₅CH₂I): This will form a benzyl carbocation (C₆H₅CH₂⁺), which is very stable due to resonance stabilization. This will also produce a yellow precipitate.
4. Conclusion: Among the options, Option B (CH₃I) is the least likely to produce a yellow precipitate with AgNO₃ due to the instability of the methyl carbocation. Therefore, it does not give a yellow precipitate.
Final Answer:
Option B (CH₃I) does not give a yellow precipitate with AgNO₃.
- Option A: CH₃CH₂I (ethyl iodide)
- Option B: CH₃I (methyl iodide)
- Option C: (CH₃)₂CHI (isopropyl iodide)
- Option D: C₆H₅CH₂I (benzyl iodide)
- Option A (CH₃CH₂I): This will form a primary carbocation (CH₃CH₂⁺), which is not very stable. However, it will still produce a yellow precipitate.
- Option B (CH₃I): This will form a methyl carbocation (CH₃⁺), which is extremely unstable and will also produce a yellow precipitate, but it is less likely to form under normal conditions.
- Option C ((CH₃)₂CHI): This will form a secondary carbocation ((CH₃)₂C⁺), which is relatively stable and will produce a yellow precipitate.
- Option D (C₆H₅CH₂I): This will form a benzyl carbocation (C₆H₅CH₂⁺), which is very stable due to resonance stabilization. This will also produce a yellow precipitate.
Option B (CH₃I) does not give a yellow precipitate with AgNO₃.
Similar Questions
Knowledge Check
Which of the following does not give yellow precipitate with NaOH+KI?
AAcetoneBAcetaldehydeCBenzaldehydeDAcetophenoneWhich of the following does not give yellow precipitate with I2 and NaOH
AC2H5OHBCH3CHOCCH3COCH3DHCHOWhich of the following will give yellow precipitate with KOI?
Aa.Cyclopentyl methyl carbinolBb.α-Phenyl ethanolCc.AAEDd.I3C−CHO
Which one of the following does not give a precipitate with AgNO3 solution ?
View SolutionWhich of the following does not form precipitate with AgNO3 ?
View SolutionWhich of the following does not form precipitate with AgNO3 ?
View SolutionWhich of the following does not form precipitate with AgNO3 ?
View SolutionWhich of the following will not give a precipitate with AgNO3 ?
View Solution
