How many orbitals, contains at least one electron in the ground state electronic configuration of Chromium atom? [Divide answer by 5]
Text Solution
Cr[24]=
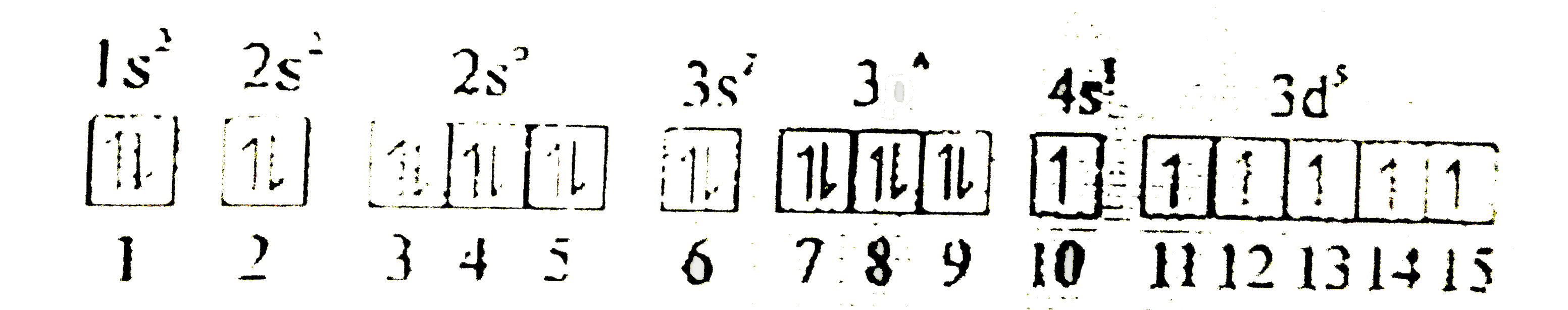
0015
|
Similar Questions
Knowledge Check
Ground state electronic configuration of Cr atom is :
A[Ar]3d44s2B[Ar]3d54s1C[Ar]3d(6)4s0D[Ar]3d54s2The ground state electronic configuration of platinum is
A[Xe]4f145d96s1B[Kr]4d105s0C[Xe]4f145d86s2D[Kr]4d45s1the ground state electronic configuration of chromium can be written as
A[Ar]3d14s1B[Ar]3d14s2C[Ar]3d54s1D[Ne]3d44s1
The correct ground state electronic configuration of chromium atom is :
View SolutionThe ground state electronic configuration of chromium is against
View SolutionThe correct state electronic configuration of chromium atom is
View SolutionThe correct ground state electronic configuration of chromium atom is :
View SolutionThe ground state electronic configuration of neutral titanium atom is
View Solution

