In Borax, hybridisation of Boron atoms are-
A
sp2,sp2
B
sp2,sp3
C
sp3,sp3
D
sp,sp3
Video Solution
Text Solution
Na2[B4O5(OH)4].8H2O
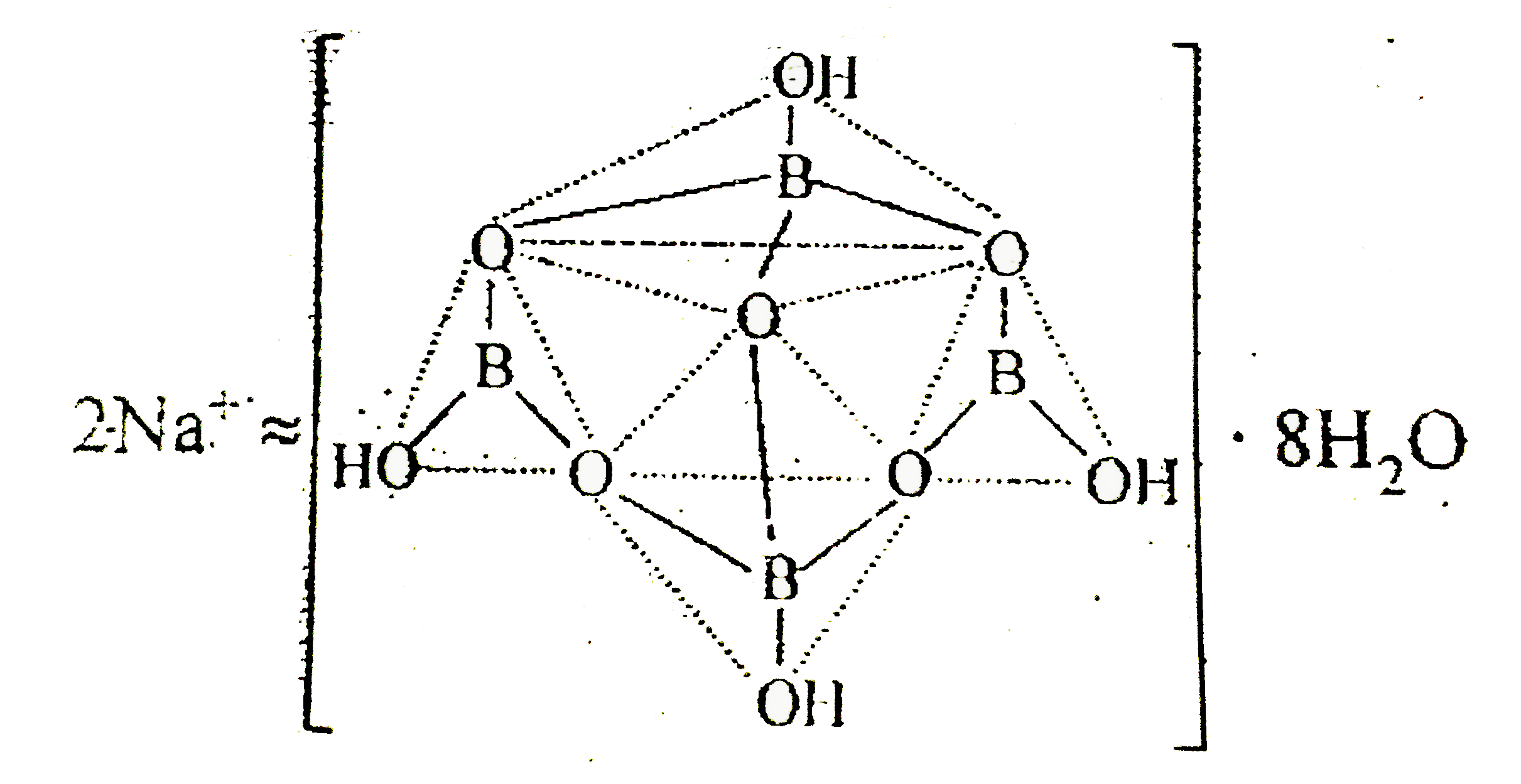
|
Similar Questions
Knowledge Check
State of hybridisation of carbon atom of carbene in the triplet state is
Asp2BspCsp3DNone of theseBorax is formulated as
Na2[B4O5(OH)4].8H2O. On the basis if its sturture answer the followings questions
Hybridisation of Boron atomsAsp3 & sp3Bsp3 & sp2Csp2 & sp2Dsp2 & spWhat is the hybridisation of boron atoms in compound Mg[B2O(OH)6] ?
ABoth sp3BOne sp2 and other sp3CBoth sp2DOne sp3 and other sp3d
Look at the figure below and find the hybridisation of carbon atom
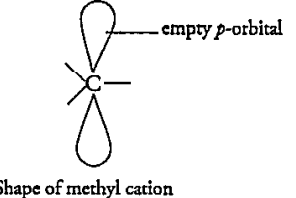
View SolutionState the hybridisation of carbon atom in carbonyl group.
View SolutionWhat is the hybridisation of carbon atoms in fullerene ?
View SolutionThe hybridisation of xenon atom XeF4 is
View SolutionThe hybridisation of boron atom in orthoboric acid is
View Solution

