K2Cr2O^(7)+H2SO4+4H2O2→
XSuphur compound+YChromium compound+5H2O
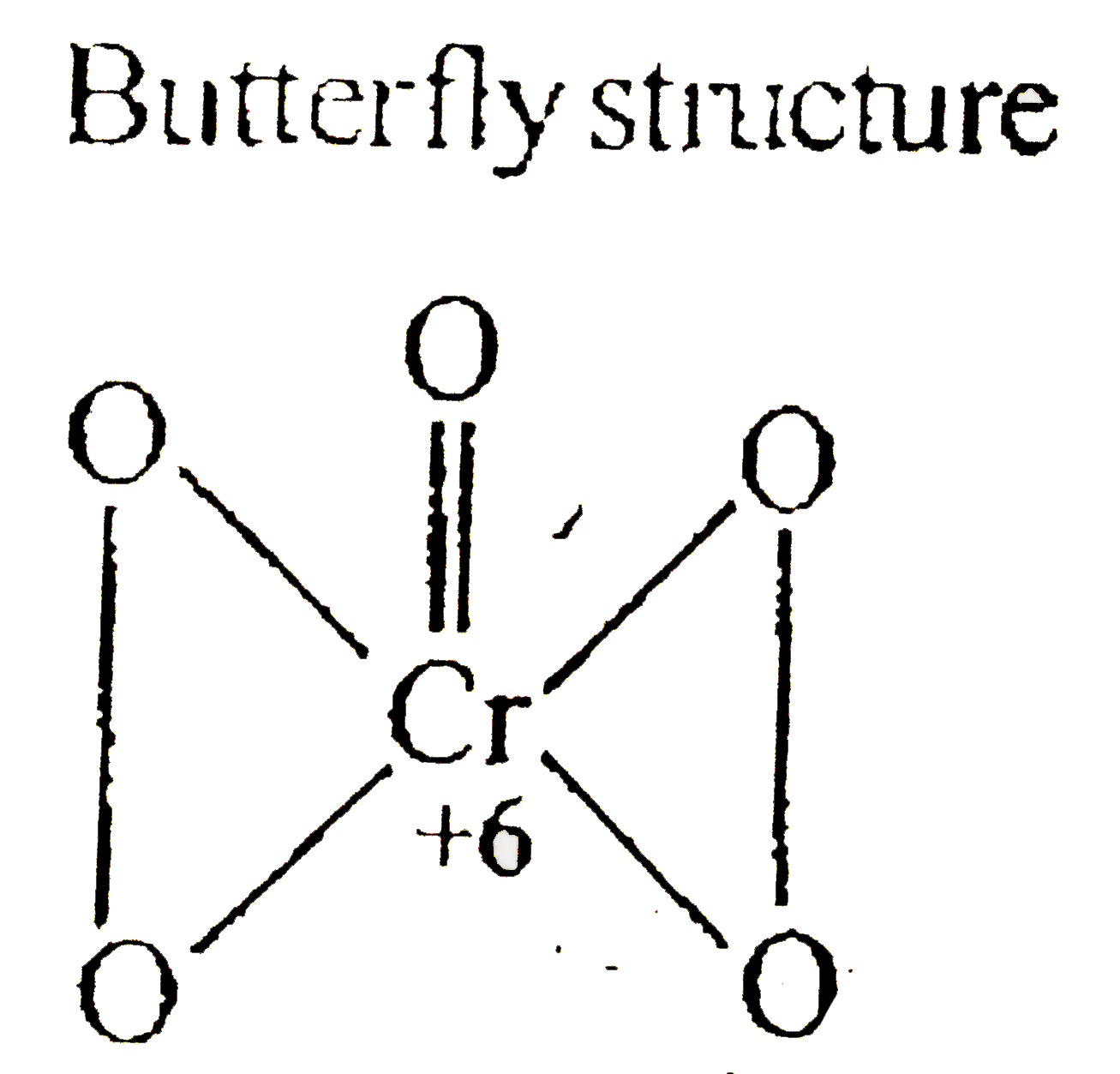
In above reaction identify the oxidation state of chrominum in compound 'Y'
A
+10
B
+8
C
+6
D
+7
Video Solution
Text Solution
Compound Y is CrO5
Butterly structure
|
Similar Questions
Knowledge Check
K2Cr2O7+SO2H+−−→products
the oxidation state of chromium changes fromA+12 to +6B+3 to +6C+6 to +3D+6 to +4K2Cr2O7+C2O2−4+H2SO4→K2SO4+CO2+Cr2(SO4)3+H2O
In above reaction, identify the elements which do not undergo change in their oxidation state :ACBS&CCK,O,S&HDC&OK2Cr2O7+H2SO4+4H2O2ether−−→K2SO4+X+H2O
The no. of oxygen atoms involved in peroxy bonds in 'X' areA1B2C3D4
NaCl(solid)+K2Cr2O7(solid)+conc.H2SO4warm−−→Reddish brown fumes of 'X'.
The oxidation state of central atom in compound 'X' is:
View SolutionNaCl(solid)+K2Cr2O7(solid)+conc.H2SO4warm−−→Reddish brown fumes of 'X'.
The oxidation state of central atom in compound 'X' is:
View SolutionToulene K2Cr2O7−−−−−→H2SO4 Y. Here Y is
View SolutionC3H8OO−−−−−−→K2Cr2O/HC3H6OI2−−−→NaOHCHI3
The starting compound is
View SolutionAK2Cr2O7−−−−−→H2SO4B[O]−−−−−−−−−−→vigorous oxidationCH3COOH If B in the above sequence is propanone, then A is
View Solution

