Identify the following reactions as disproportionation or comproportionations reactions-
4KClO3(s)→KCl(s)+3KClO4(s)Solution in Bengali
Video Solution
Text Solution
4K+5CIO3(s)→KC−1I+3K+7CIO4(s). In this reaction the oxidation number of CI decreases (+5to -1) as well as increases (+5 to+7). This means in the reaction .Hence the reaction is a disproportionation reaction.
|
Similar Questions
Identify the following reactions at disproportionation a comproportionations reactions-
2MnO44(aq)+2H2O(l)→2MNO−4(aq)+MnO2(s)+4OH−(aq)
View SolutionIdentify the following reactions at disproportionation a comproportionations reactions-
2NH4NO3(s)→N2(g)+4H2Og+O2(g)
View SolutionIdentify the following reactions as disproportionation or comproportionations reactions- IO_(3)-(aq)+5I^(-)(aq)+6H^(+)(aq)to3I_(2)(s)+3H_(2)O(l)
View SolutionWhich of the following reaction are disproportiontion reaction and comproportionaton reactions ? 4KClO_(3)toKCl+3KClO_(4)
View SolutionDistinguish between disproportionation reactions and comproportionation reactions.
View SolutionWhich is (are) disproportionation reaction(s)?
View Solution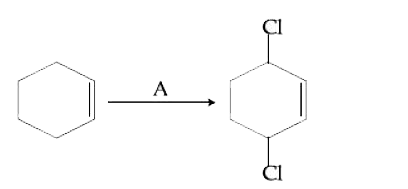
Identify the reagent (s) 'A' and condition (s) for the reaction
View Solution

