Assign oxidation number to the underline elements in each of the following species:
NaH2p–O4Solution in Bengali
Video Solution
Text Solution
Let the oxidation number P in NaH2PO4 be x ∴1(+1)+2(ForNa)+1(ForH)+x+4(−2)(ForO)=0∴x=+5
|
Similar Questions
Assign oxidation number to the underline elements in each of the following species: KAIunderline((S)O_(4))(2).12H_(2)O
View SolutionAssign oxidation number to the underlined elements in each of the following species
View SolutionAssign oxidation number to the underlined elements in each of the following species
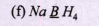
View SolutionAssign oxidation number to the underlined element in the following species
View SolutionAssign oxidation number to the underlined elements in each of the following species :
NaH2P––O4
View SolutionAssign oxidation number to the underlined elements in each of the following species :
NaHS––O4
View SolutionAssign oxidation number to the underlined elements in each of the following species :
H4P––2O7
View SolutionAssign oxidation number to the underlined elements in each of the following species : K_(2)ul(Mn)O_(4)
View Solution
