A gas (γ=1.5) undergoes a cycle of adiabatic, isobaric and isochoric processes in an order. If the volume of the gas is doubled in the adiabatic process then the efficiency of the cycle is approximately,
18%
46.4%
38.5%
9.25%
The correct Answer is:A
According to the question, we drawn the following situation,
In isochoric process, pATA=pBTB
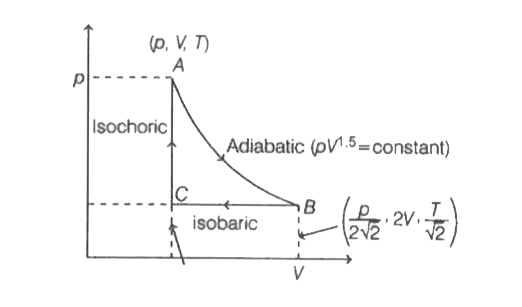
As work done in adiabatic process,
W=nR(T1−T2)y−1=R(T−T√2)(1.5−1) (Taking, n=1)
V2=2V1
⇒ T2=T1√2 or T2=T√2
Wt=RT2(1−1√2)=0.58 (∵r=i Given )
Work done in isobaric process,
W2=pΔV=nRΔT
⇒W=nR(T2√2−T√2)=−RT(1√2−12√2)=−0.35
Now, heat given to system
Q1=nCpΔT=72R(1√2−12√2)=−1.23J
Negative, Q1 shows that heat is given to the system.
Efficiency of the cycle,
η=net work doneheat input=W1+W2Q1
=0.58−0.351.23
⇒ η=0.231.23×100=18.6%=18%
Hence, the efficiency of cycle is 18%.
Similar Questions
The efficiency of an ideal gas with adiabatic exponent 'gamma' for the shown cyclic process would be
View SolutionTwo moles of an ideal gas goes under the process shown in figure. AB and CD are reversible adiabatic process. The efficiency of the cycle is
View SolutionWhat are isobaric, isochoric, isothermal and adiabatic processes?
View SolutionAn ideal gas undergoes four processes: isochoric, isobaric, isothermal and adiabatic. In which process is (i) the change in internal energy zero?
View SolutionAn ideal gas undergoes four processes: isochoric, isobaric, isothermal and adiabatic. In which process is (ii) the work done zero?
View SolutionAn ideal gas undergoes four processes: isochoric, isobaric, isothermal and adiabatic. In which process is (iii) the heat exchange zero?
View SolutionThe efficiency of an ideal gas with adiabatic exponent 'γ' for the shown cyclic process would be
View Solution

