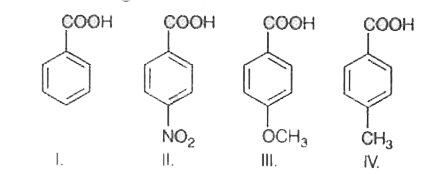
The increasing order of acidity of the following carboxylic acids is
 Solution in Telugu
Solution in Telugu
III>IV>II>I
II>III>II>I
I>II>IV>III
III>IV>I>II
The correct Answer is:D
(i) −NO2 group show -l effect thus increases the acidic nature
(ii) −OCH3andCH3 are electron donating groups, thus decreases the acidic nature. But −OCH3 will decrease acidic nature more than of −CH3 group
Hence, correct order is
c<d<a<b
Similar Questions
अभिकथनः समूहों का प्रत्यक्ष जुड़ाव जैसे फिनाइल या विनाइल का कार्बोक्जिलिक अम्ल से, कार्बोक्जिलिक अम्ल की अम्लीयता को बढ़ाता है।
तर्कः अनुनाद प्रभाव हमेशा कार्बोक्सिलिक अम्लों की अम्लीयता को बढ़ाता है।
View SolutionArrange the following carboxylic acids in order of increasing acidity. m-Nitrobenzoic acid, Trichloroacetic acid, benzoic acid, alpha-Chlorobutyric acid.
View SolutionThe correct order of acidic strength for the following carboxylic acid is
View SolutionThe correct order of an acidic nature of following Carboxylic acids is
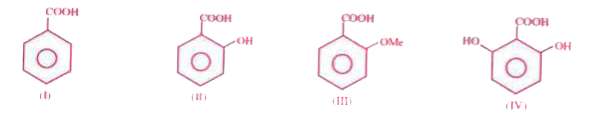
View SolutionThe inceasing order of the acidity of the following carboxylic acids is :
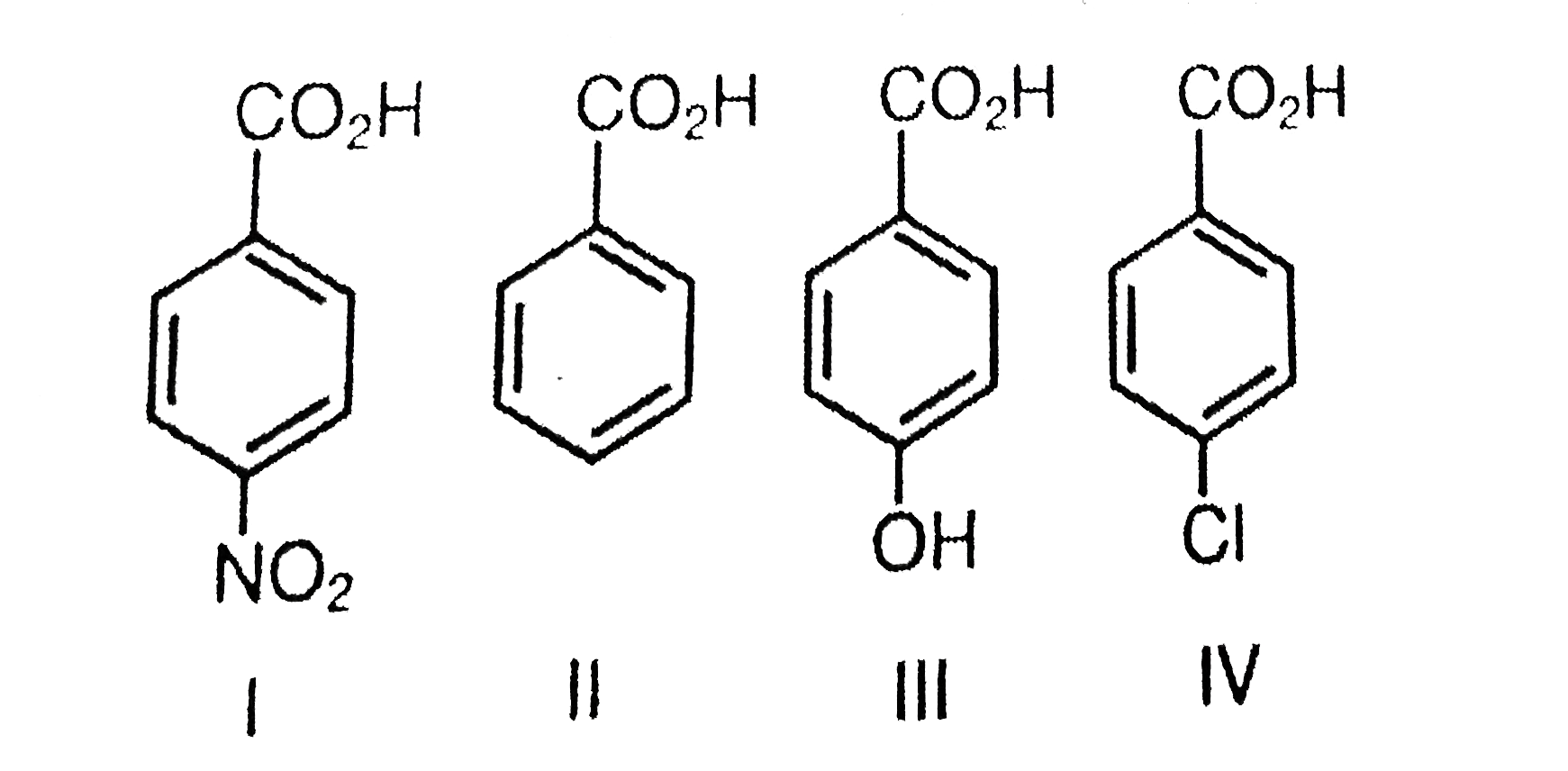
View SolutionThe correct order for the acidic character of the following carboxylic acids is :
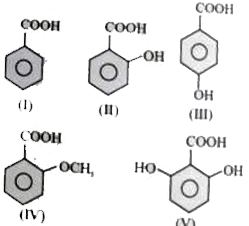
View SolutionConsider the acidity of the carboxylic acids Which of the following order is correct?
View Solutionनिम्न कार्बोक्सिलिक अम्ल व्युत्पन्नों को उनकी बढ़ती हुई अम्लीयता के क्रम में लिखिए।
View Solution
