In how many of the following compounds of sulphur, the oxidation state of sulphur atom is + 6 ?
H2S2O8,H2SO5,H2SO3,H2SO4,H2S2O7,SO2CL2,SOCL2Solution in Telugu
3
5
4
6
The correct Answer is:C
The oxidation state of sulphur in the given compounds can be found through their respective structures which are as follows :
(a) H2S2O8
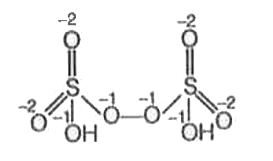
We can calculate oxidation state sulphur as :
x+2(−2)+1(−1)+1(−1)=0
x = + 6
Thus, the sulphur is in + 6 oxidation state .
(b). H2SO3
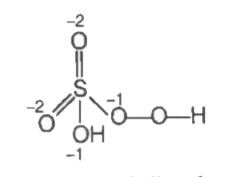
∴x+2(−2)+1(−1)+1(−1)=0
x = + 6
Thus, the sulphur is in + 6 oxidation state.
(c) H_(2) SO_(3)
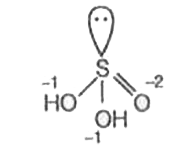
x + 2 (-1) + 1 (-2) = 0
x = + 4
Thus, sulphur is present in + 4 oxidation state.
(d) H2SO4
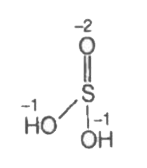
x + 2 (-1) + 1 (-2) = 0
x = + 4
Thus, sulphur is present in + 4 oxidation state.
(e) H2S2O7
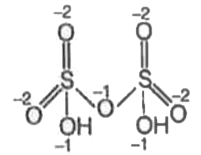
x + 2 (-2) + 1 (-1) + 1 (-1) = 0
x = + 6
Thus, sulphur is present in + 6 oxidation state.
(f) SO2Cl2
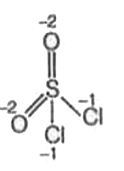
x + 2 (-1) + 2(-2) = 0
x = + 6
Thus , sulphur has + 6 oxidation state.
(g) SOCl2
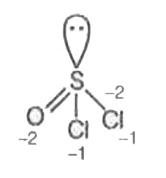
x + 1 (-2) + 2 (-1) = 0
x = + 4
Thus, sulphur, has 4 oxidation state,
Compounds having+ 6 oxidation state of sulphur atom are H2S2O2,H2SO5,H2S2O7,SO2Cl2
Hence, the total number of molecules having + 6 oxidation state of sulphur atom is 4 .
Similar Questions
Oleum is a mixture of \(H_{2}SO_{4}\)+\(SO_{2}\) \(H_{2}SO_{4}\)+\(SO_{3}\) \(H_{2}S_{2}O_{3}\)+\(SO_{3}\) \(H_{2}S_{2}O_{3}\)+\(SO_{2}\)
View SolutionThe oxidation number of sulphur atoms in peroxomonosulphuric acid (H_(2)SO_(5)) and peroxodi - sulphuric acid (H_(2)S_(2)O_(8)) are respectively
View SolutionThe oxidation number of sulphur atoms in peroxomonosulphuric acid (H2SO5) and peroxodi - sulphuric acid (H2S2O8) are respectively
View SolutionOxidation state of sulphur in \(H_{2}S_{2}O_{8}\) and \(H_{2}SO_{5}\)
View SolutionOxidation state of S in H_(2)SO_(5) and H_(2)S_(2)O_(8) respectively are
View SolutionCalculate the oridation numbers of sulphur in H2SO5 and in H2S2O8
View SolutionCalculate the oxidation number of sulphur in H2SO3 and in H2S2O8
View SolutionThe oxidation numbers of the sulphur atoms in pcroxy- monosulphuric acid (H2SO5) and peroxydisulphuric acid (H2S2O8) are respectively.
View Solution
