A glass tube of small bore is sealed at both ends,inside the tube a mercury thread is so confined that it divides the length of the tube in the ratio of 3:1 IF the temperature of the whole system is raised from 0∘C to 273∘C, them what will be the observed change in the pressure of air columns inside the tube?
The correct Answer is:pressure at each part will be doubled
Topper's Solved these Questions
- EXPANSION OF GASESBOOK - CHHAYA PUBLICATIONCHAPTER - EXPANSION OF GASESEXERCISE - ENTRANCE CORNER(Assertion -Reason type)
4 Videos
- EXPANSION OF GASESBOOK - CHHAYA PUBLICATIONCHAPTER - EXPANSION OF GASESEXERCISE - ENTRANCE CORNER(Multiple correct answer type)
3 Videos
- EXPANSION OF GASESBOOK - CHHAYA PUBLICATIONCHAPTER - EXPANSION OF GASESEXERCISE - EXERCISE ( Problem set -II)
10 Videos
- ELASTICITY BOOK - CHHAYA PUBLICATIONCHAPTER - ELASTICITY EXERCISE - CBSE SCANNER
10 Videos
- EXPANSION OF SOLIDS AND LIQUIDSBOOK - CHHAYA PUBLICATIONCHAPTER - EXPANSION OF SOLIDS AND LIQUIDSEXERCISE - CBSE SCANNER
2 Videos
Similar Questions
A long horizontal glass capillary tube open at both ends contains a mercury thread 1 m long at 0∘C. Find the length of the mercury thread, as read on this scale, at 100∘C.
View SolutionA long horizontal glass capillary tube open at both ends contains a mercury thread 1 m long at 0∘C. Find the length of the mercury thread, as read on this scale, at 100∘C.
View Solution
Knowledge Check
A horizontal uniform glass tube of 100cm length is sealed at both ends contains 10cm mercury column in the middle , the temperature and pressure of air on either side of mercury column are repectively 31∘C and 76cm of mercury, if the air column at one end is kept at0∘C and the other end at 273∘C then pressure if air which is 0∘C is
(in cm of Hg)A76B88.2C102.4D122A thin tube of uniform cross-section is sealed at both ends. It lies horizontally, the middle 5 cm containing mercury and the two equal end containing air at the same pressure P. When the tube is held at an angle of 60∘ with the vetical direction, the length of the air column above and below the mercury column are 46 cm and 44.5 cm respectively. Calculate the pressure P in centimeters of mercury. (The temperature of the system is kept at 30∘C).
A75.4B45.8C67.5D89.3
A glass tube sealed at one end and containing a quantity of air is immersed in mercury until the sealed end is 10 cm from the surface of mercury. At 0∘C the level of mercury in the tube is 5 cm above the level of mercury in the vessel. The length of the tube is 15 cm. To what temperature should the air in the tube be raised as to fill the tube completely? The atmospheric pressure is 75 cm of Hg. Neglect any change in the level of mercury in the vessel.
View SolutionA horizontal tube of 1m is closed at both ends. A mercury column of 20 cm is contained in the middle of the tube and two equal lengths of this tube contain air at an atmospheric pressure of 76cm. The tube is now placed vertically. Calculate the distance through which the mercury column will be displaced at a constant temperature.
View SolutionA horizontal uniform glass tube of 100cm length is sealed at both ends contains 10cm mercury column in the middle , the temperature and pressure of air on either side of mercury column are repectively 31∘C and 76cm of mercury, if the air column at one end is kept at0∘C and the other end at 273∘C then pressure if air which is 0∘C is
(in cm of Hg)
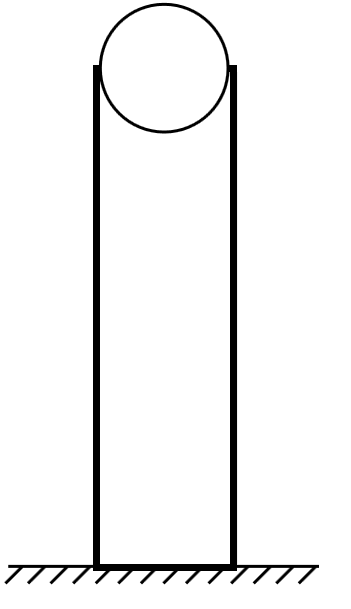
View SolutionThere is a long vertical tube of radius r containing air at atmospheric pressure. A steel ball is held at the mouth of the tube and dropped. The ball has radius r and it just fits inside the tube. The tube wall is perfectly smooth and no air can leak from the tube as the ball falls inside it. The ball falls through half the length of the tube before coming to rest. Assume that wall of the tube is perfectly conducting and temperature of the air inside the tube remains constant. Density of steel is d and atmospheric pressure is P0 and take L>>r. Take air to be an ideal gas.
(a) Find the radius (r) of the tube.
(b) At what depth from the top of the tube the ball will be in equilibrium?
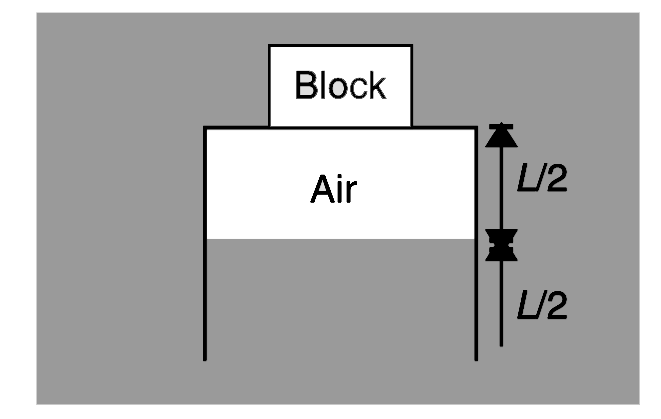
View SolutionA light cylinderical tube of length L=1.5m and radius v=1√πm is open at one end. The tube containing air is inverted and pushed inside water as shown in figure. A block made of material of relative density 2 haas been placed on the flat upper surface of the tube and the whole system is in equilibrium Neglect the weight of air inside the tube and find the volume of block placed on the tube.
View SolutionA glass tube with a sealed end is completely submerged in a vessel with mercury. The air column is 15 cm long. The what height (in cm) must the upper end be raised above the level of Hg so that the level of Hg inside the tube is at the level of Hg in the veseel ?
[Atmospheric pressure = 75 cm of Hg column]
View Solution

