In a process, if dU, dW and dQ are change in internal energy, work done and heat accepted respectively for a system, what is the relation between dU , dQ and dW?Solution in Bengali
dQ=dU+dW
Similar Questions
Suppose 300J of work done on a system and 70cal of heat is extracted from the system. What are the values of dW,dQanddU with proper signs?
View SolutionFirst law of thermodynamics is the general law of conservation of energy as applied to heat energy. According to this law, whenever heat is added to a system, it transforms to an equal amount of some other forms of energy. If dQ= a small amount of heat supplied to the system, dW= small amount of work done by the system and dU= small change in internal energy of the system, then dQ=dU+dW . Read the above passage and answer the following questions: (i) The amount of heat obsorbed by a gas is 10 cals and work done on the gas in 58J . Calculate increase in internal energy of the gas. (ii) What values of life do you learn fron this law?
View SolutionIn the relation dQ=dU+dW , the quantity which remains the same for all process is
View SolutionIn an isobaric process, the ratio of heat supplied to the system (dQ) and work done by the system (dW) for diatomic gas is
View SolutionFigure shows the P−V siagram of a cyclic process. If dQ is the heat energy supplied to the system, dU is change in the internal energy of the system and dW is the work done by the system, then which of the following relations is/are correct
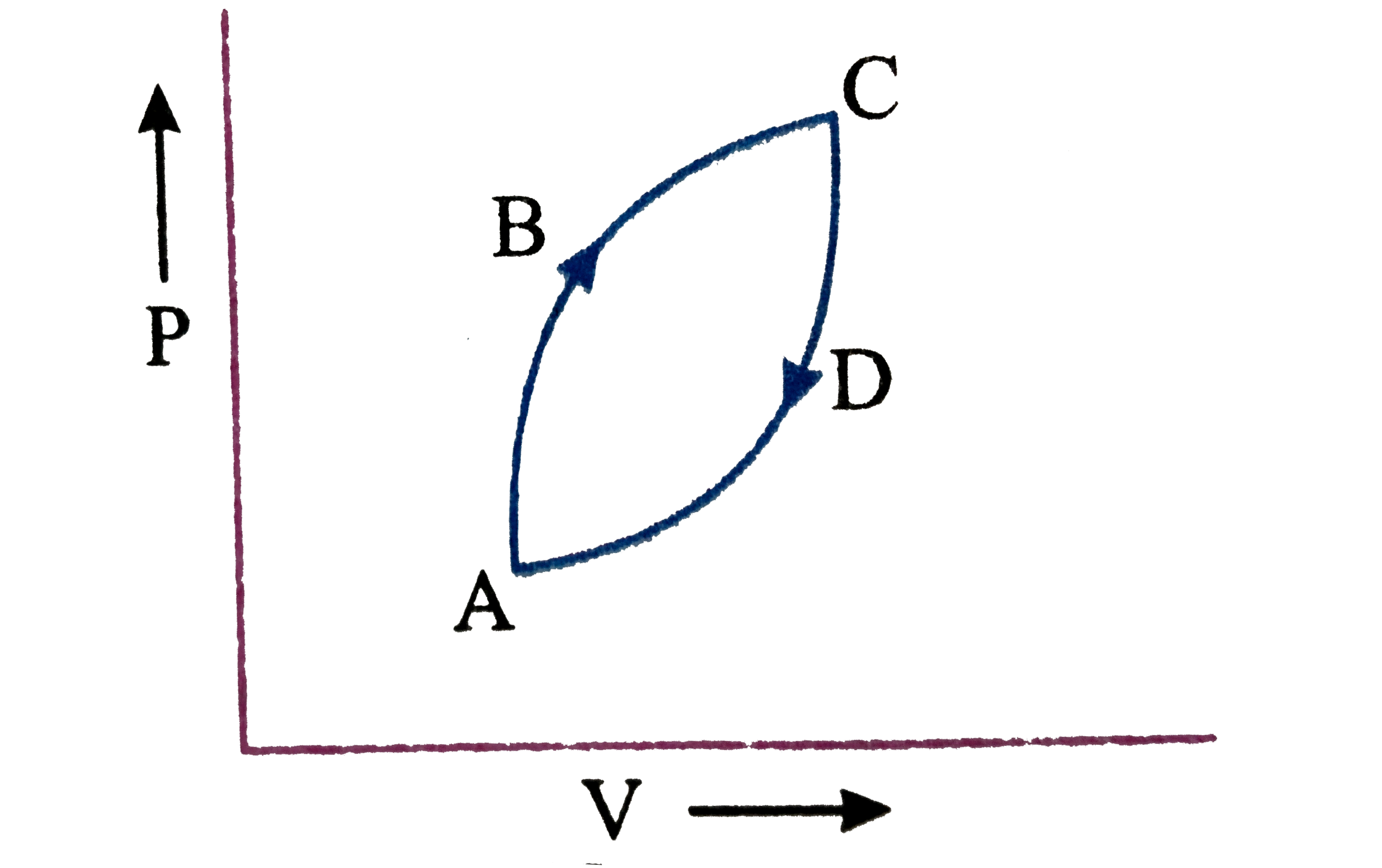
View SolutionA thermodynamic process obeys the following relation 2dQ=dU+2dW where dQ, dU, dW has ussual meaning then at which statement is true [given di-atomic gas, R=gas constant] then heat capacity for the process is
View SolutionIn an isobaric process, the ratio of heat supplied to the system (dQ) and work done by the system (dW) for diatomic gas is
View SolutionA sample of an ideal gas undergoes on isothermal expansion. If dQ, dU and dW represent the amount of heat supplied, the change in internal energy and the work done respectively,then
View SolutionExternal work done by an ideal gas during Expansion dw is (A) v dv (B) p du (C) p dv (D) dQ
View SolutionExplain the relation dH = dU + dW .
View SolutionIdeal monoatomic gas is taken through a process dQ=2dU . What is the molar heat capacity for the process ? (where dQ is heat supplied and dU is change in internal energy)
View SolutionWhen a gas is supplied 'dQ' heat, it performs a work 'dW' . The increase in its internal energy 'dU' is
View SolutionAn ideal gas undergoes isothermal process from some initial state I to final state f. Choose the correct alternatives.
a) dU=0
b) dQ=0
c) dQ=dU
d) dQ=dW
View SolutionIn the equation for I law of thermodynamics dQ=du+dw
a) dQ depends on path
b) dw depends on path
c) du depends on path
View SolutionList -IList -II(a)Isobaric process (e)dQ=dw(b)Isochoric process(f)dQ=dU+dw(c)Isothermal process (g)dU=−dw(d)Adiabatic process(h)dQ=dU
View Solution
