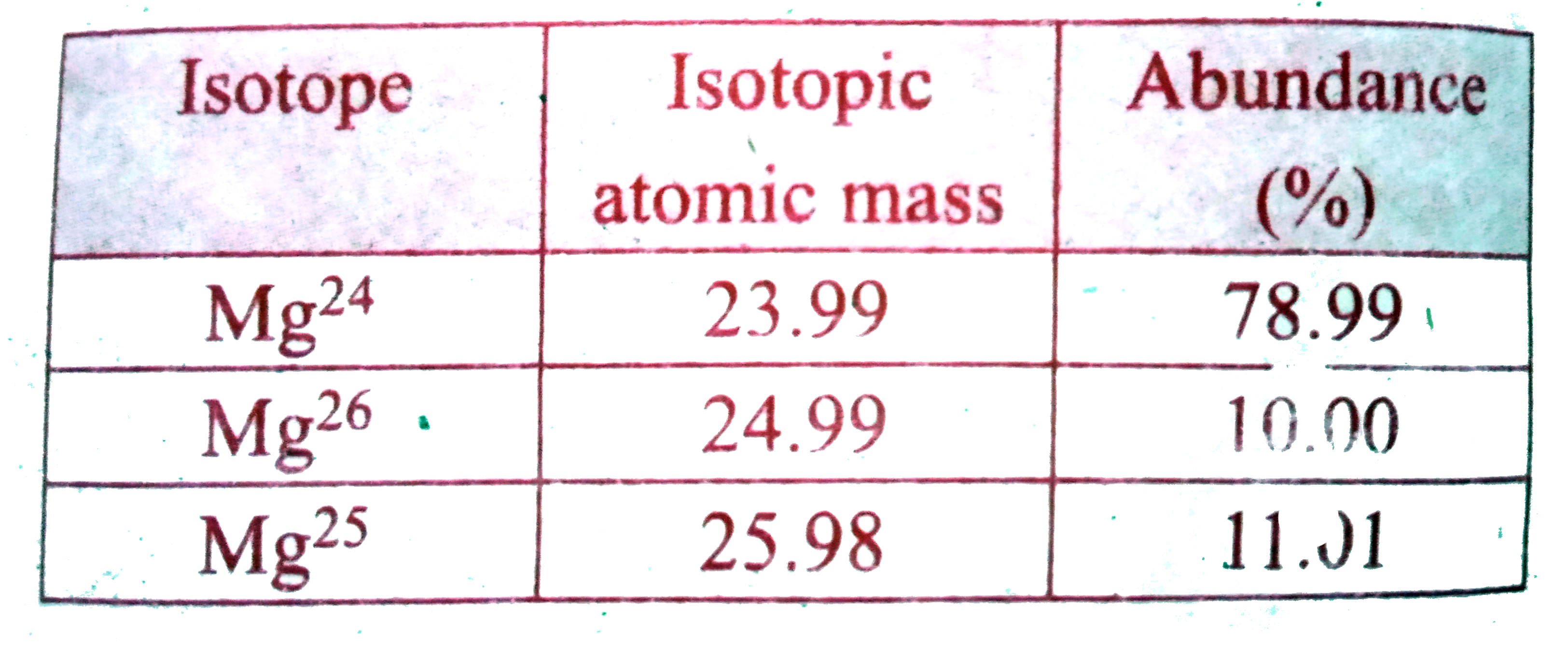
Calculate the average atomic mass of naturally occurring magnesium using the following data

Isotopes of Mg
Atomic mass =Mg24=23.99×78.99100=18.95
Atomic mass =Mg25=24.99×10100=2.499
Atomic mass =Mg26=25.98×11.01100=2.860
Average atomic mass =24.309
Average atomic mass of Mg =24.309.
Similar Questions
निम्नलिखित आँकड़ों का उपयोग करके हाइड्रोजन के औसत परमाणु द्रव्यमान की गणना कीजिए
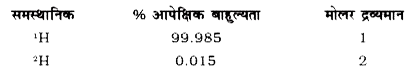
View SolutionCalculate the atomic mass (average) of chlorine using the following data :
View Solutionuse the data given in the following table to calculate the molar mass of naturally occuring argon isotopes.
View SolutionCalculate the atomic mass (average) of chlorine using the following data:
View SolutionThe average molar mass of naturally occurring Argon. Calculated using the following data is
View SolutionThe average mass of one gold atom in a sample of naturally occuring gold is 3.2707×10−22g. Use this to calculate the molar mass of gold.
View SolutionThe average mass of one gold atom in a sample of naturally occuring gold is 3.2707×10−22g. Use this to calculate the molar mass of gold.
View SolutionCalculate the average atomic mass of naturally occurring magnesium using the following data
View Solution

