What are the significance of ΨandΨ2 ?
Text Solution
In an atom the wave function Ψ for an electron has no physical significance as such. However, its square i.e., Ψ2 at any point gives the intensity of the electron wave at that point.
In view of Heisenberg.s uncertainty principle, it shows the probability of finding the electron at that point and therefore, termed as probability density.
|
Similar Questions
Knowledge Check
The significance of |Ψ|2x is
AProbabilityBEnergyCProbability densityDEnergy density
What is the significance of the sign ψ2 ?
View SolutionMention the physical significance of ΨandΨ2
View SolutionWhat is the significance of ψ210 ? Find out angular momentum , spherical nodes and angular node for ψ210.
View SolutionWhat does Ψ2 indicate?
View SolutionOrbitals are the pictorial representationofΨ or Ψ2

Ψ2 tell about the probability of finding electron.
Which of the following graph is correct for 3p ?
View SolutionOrbitals are the pictorial representationofΨ or Ψ2
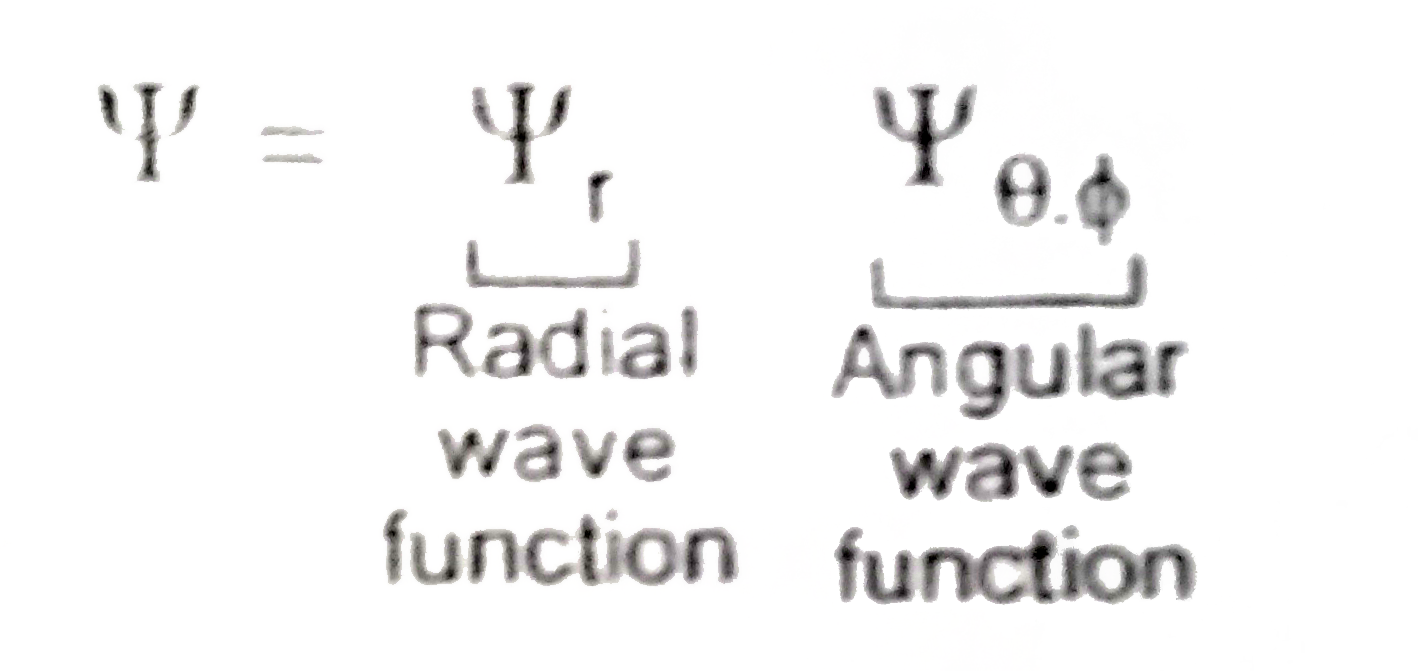
Ψ2 tell about the probability of finding electron.
Which of the following orbital is non directional ?
View SolutionOrbitals are the pictorial representationofΨ or Ψ2
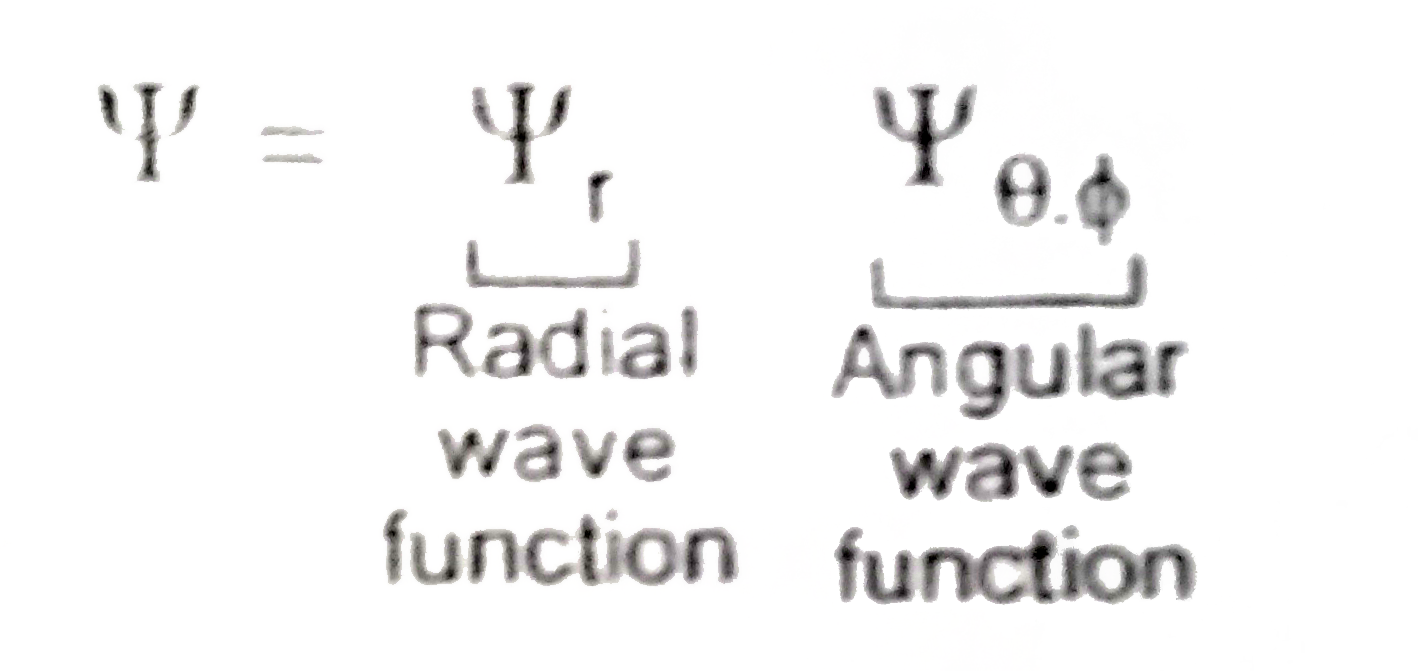
Ψ2 tell about the probability of finding electron.
Which of the following orbital is non directional ?
View Solution

