Write the Born-Haber cycle for NaCl.Solution in Kannada
Video Solution
Text Solution
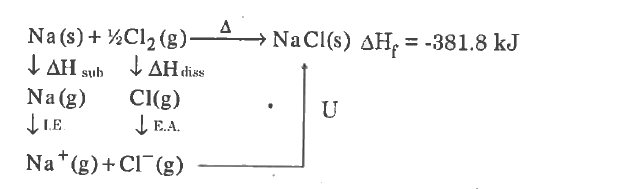
When U= Littice energuy :ΔHs = Enthaply of sublimation, = Enthalpy of ionization, ΔH4= enthalpy of dissociatio, ΔHi=ΔHEa enthalpy for electron affinity. The only one step form formation of NaCl from sodium cholorine is associated with the energy called ΔHf = = enthalpy of formation.
∴ΔHf=U+ΔHs+ΔHi+ΔHdΔHEa
∴ Lattice energy U=DE<aHf=[ΔHs+ΔHi+ΔHd+ΔHEa]
|
Similar Questions
Born Haber cycle is based on
View SolutionCalculate the lattice energy of NaCl using Born-Haber cycle.
View SolutionBorn-Haber cycle is related with ___________.
View SolutionCalculate the lattice energy of NaCl using Born-Haber cycle.
View SolutionBorn-Haber cycle is related with ___________.
View SolutionWrite notes on: Born-Haber Cycle.
View SolutionWhich of the following is INCORRECT regarding Born-Haber cycle for the formation of NaCl?
View SolutionBorn Haber Cycle
View Solution

