Based on VSEPR theory explain the structure of methane. Solution in Kannada
Video Solution
Text Solution
In the formation of methane, carbon utilizes sp3 hybrid orbitals for bonding. The four sp3 hybrid orbitals of carbon atom overlaps axially with a orbitals of four hydrogen atoms to give four C−H s bond. The C-H s bond is sp3-s bond. H-C-H bond angle is 109∘28. the shape of the molecule is tetrahedral.
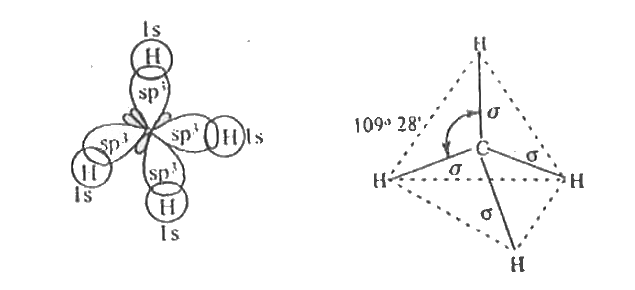
|
Similar Questions
Apply VSEPR theory to deduce the structures of XeF_4 and XeF_6
View SolutionVSEPR सिद्धान्त के आधार पर XeOF2 की आकृति होगी
View SolutionVSEPR theory is used to predict the shape of covalent molecules .
b) Based on VSEPR theory predict the shape of H2O and NH3.
View SolutionThe shapes of the molecules is based on the VSEPR theory. Give the salient features of this theory
View SolutionDraw the structure of XeF2, XeF4 and XeF6.
View SolutionDiscuss the structure of the XeF_4 on the basis of VSEPR theory.
View SolutionExplain the postulates of VSEPR theory.
View SolutionExplain VSEPR theory .appling this theory to predict the shapes of IF7 and SF6
The following are the basis of VSEPR theory :
View Solution

