Complete the following chemical equations.
Cu+2H2SO4→CuSO4+____________+2H2OSolution in Kannada
Cu+2H2SO4→CuSO4+SO2+2H2O
Topper's Solved these Questions
- ANNUAL EXAM QUESTION PAPER WITH ANSWER MARCH(2016)Book:SUBHASH PUBLICATIONChapter:ANNUAL EXAM QUESTION PAPER WITH ANSWER MARCH(2016)Exercise:PART D
Explore 11 Videos
- ANNUAL EXAM QUESTION PAPER WITH ANSWER MARCH(2016)Book:SUBHASH PUBLICATIONChapter:ANNUAL EXAM QUESTION PAPER WITH ANSWER MARCH(2016)Exercise:PART E
Explore 16 Videos
- ANNUAL EXAM QUESTION PAPER WITH ANSWER MARCH(2016)Book:SUBHASH PUBLICATIONChapter:ANNUAL EXAM QUESTION PAPER WITH ANSWER MARCH(2016)Exercise:PART B
Explore 9 Videos
- ANNUAL EXAM QUESTION PAPER MARCH 2017Book:SUBHASH PUBLICATIONChapter:ANNUAL EXAM QUESTION PAPER MARCH 2017Exercise:PART D
Explore 11 Videos
- ANNUAL EXAMINATION QUESTION PAPER MARCH - 2018Book:SUBHASH PUBLICATIONChapter:ANNUAL EXAMINATION QUESTION PAPER MARCH - 2018Exercise:V. Answer any four of the following . Each question carriers five marks.
Explore 17 Videos
Similar Questions
Calculate the number of particles in Body Centered Cubic (BCC) lattice.
View SolutionExplain the mechanism of SN1 reaction taking 2-bromo-2-methyl propane (t-butyl bromide)
View SolutionCalculate the no. of particles (atoms) per unit cell in a FCC crystal lattice:
View SolutionAn element having atomic mass 63.1 g/mol has face centered cubic unit cell with edge length 3.608×10−8 cm. Calculate the density of unit cell [Given NA=6.022×1023 atoms/mol].
View SolutionWrite the equations for the steps in SN1 mechanism of the conversion of the conversion of tert -butyl bromide into tert-butyl alcohol.
View SolutionComplete and balance the following equations:
CuS+2H2SO4+….→2CuSO4+2H2O+S
View Solutionनिम्न रासायनिक समीकरण को पूर्ण कीजिएः
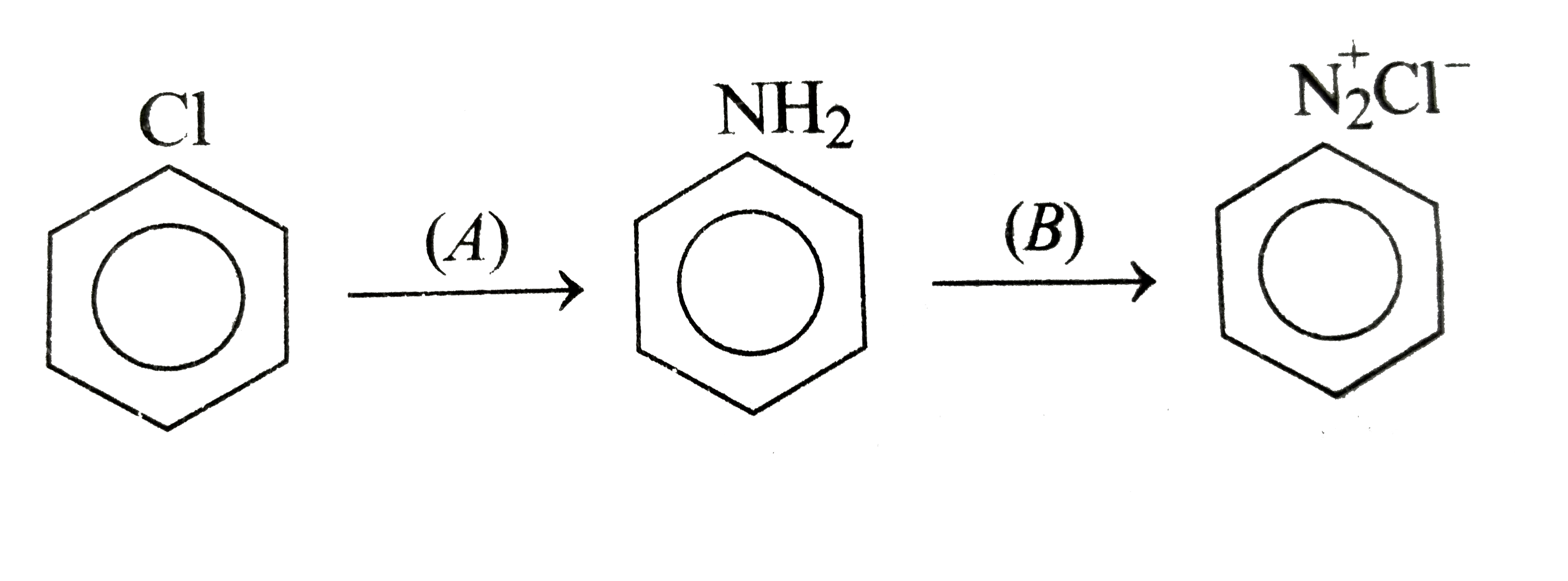
View Solutionनिम्नलिखित रासायनिक समीकरण को पूर्ण कीजिए-

View SolutionComplete the following chemical equations. Cl_2+2H_2O+SO_2to"________"+2HCl
View SolutionComplete the following chemical equations : Cu^(2+) (aq) + NH_3 (aq) to
View SolutionComplete the following chemical equations :
Cu+HNO3 ( dilute) →
View SolutionThe equation Cu + XHNO_3 to Cu(NO_3) + YNO_2 + 2H_2O the values of X and Y are
View SolutionComplete and balance the chemical equation: CaC_2 + 2H_2O to "_____" + Ca (OH)_2
View SolutionComplete and balance the chemical equation: "_____" + 5O_2 to 4CO_2 + 2H_2O + heat
View SolutionComplete the followign chemcial equations: 2XeF2+2H2O→
View Solution
