(a) Write the structures of A and B in the following reactions :
(i) CH3COClH2,Pd−BaSO4−−−−−−−−−→AH2N−OH−−−−−−→B
(ii) CH3MgBr1.CO2−−−−−−−→2.H3O+APCl5−−→B
(b) Distinguish between :
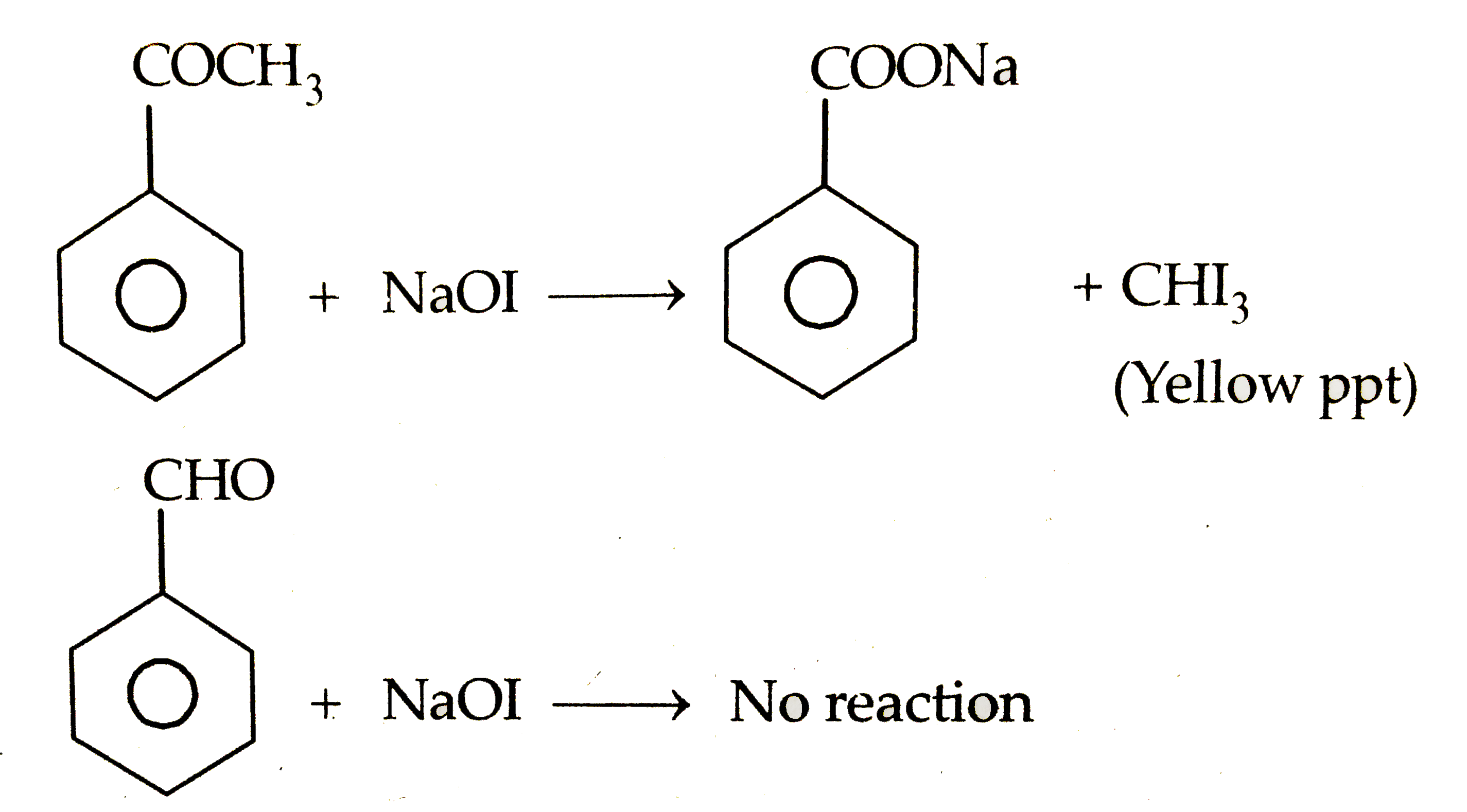
(i) C6H5−COCH3 and C6H5−CHO
(ii) CH3COOH and HCOOH
(c) Arrange the following in the increasing order of their boiling points :
(i) CH3CHO,CH3COOH,CH3CH2OH
(a) (i) 
(ii) CH3MgBrCO2−−−−−−→H3O+CH3COOH(A)PCI−−→CH3C∣∣C(B)−CI
(b) (i) Acetophenone and Benzaldehyde
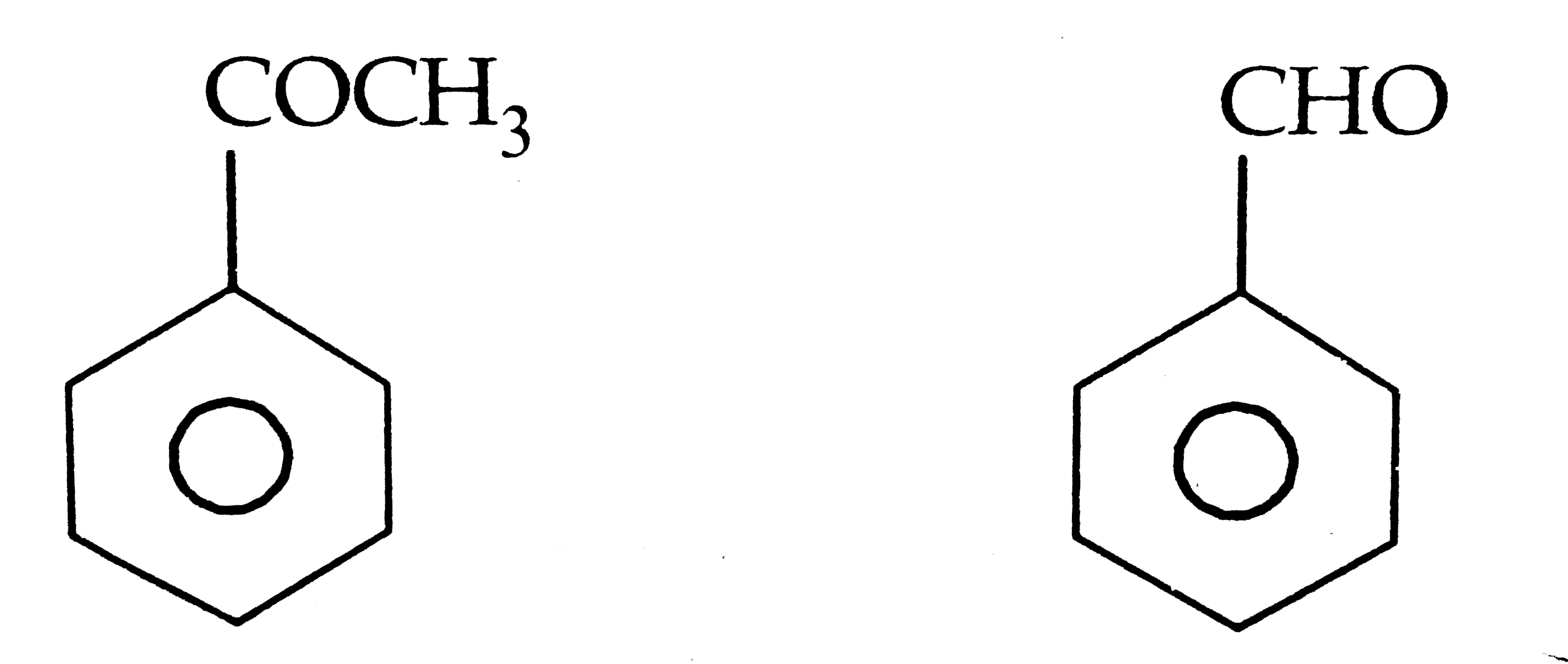
Iodoform test
(ii) CH3COOH and HCOOH
Formic acid decolourises the Pink colour of acidified KMnO4 solution, while acetic acid does not give this lest.
5HCOOH+2KMnO4+3H2SO4→K2SO4+5CO2+8H2O
CH3COOHKMnO4/H+−−−−−−−−→ Does not discharge pink colour of KMnO4 solution.
(c) CH3COOH>CH3CH2>OH>CH3CHO
Reason : Because of stronger hydrogen bonding in acid the boiling point of carboxylic acids of comparable molecular mass is higher than that of alcohols.
Similar Questions
Knowledge Check
CH3COCl+H2Pd−−−→BaSO4CH3CHO+HCl is:
ACross aldol condensationBAldol condensationCCannizzaro reactionDRosenmund's reaction
Distinguish between : (ii) C6H5COCH3andC6H5CH2CHO.
View SolutionWrite the structure of A,B,C and D in the following reactions:
CH3COOHPCl5−−→AH2/Pd−BaSO4−−−−−−−−−→BLiAlH4DCH3MgBr−−−−−→C
View SolutionIdentify A and B in the following reactions :
C6H5CHO+CH3−CH2−CO−CH3−OH−−−−−→A−H2O−−−→BI2/NaOH−−−−−→C
View Solution(a) Complete the following equations :
(i)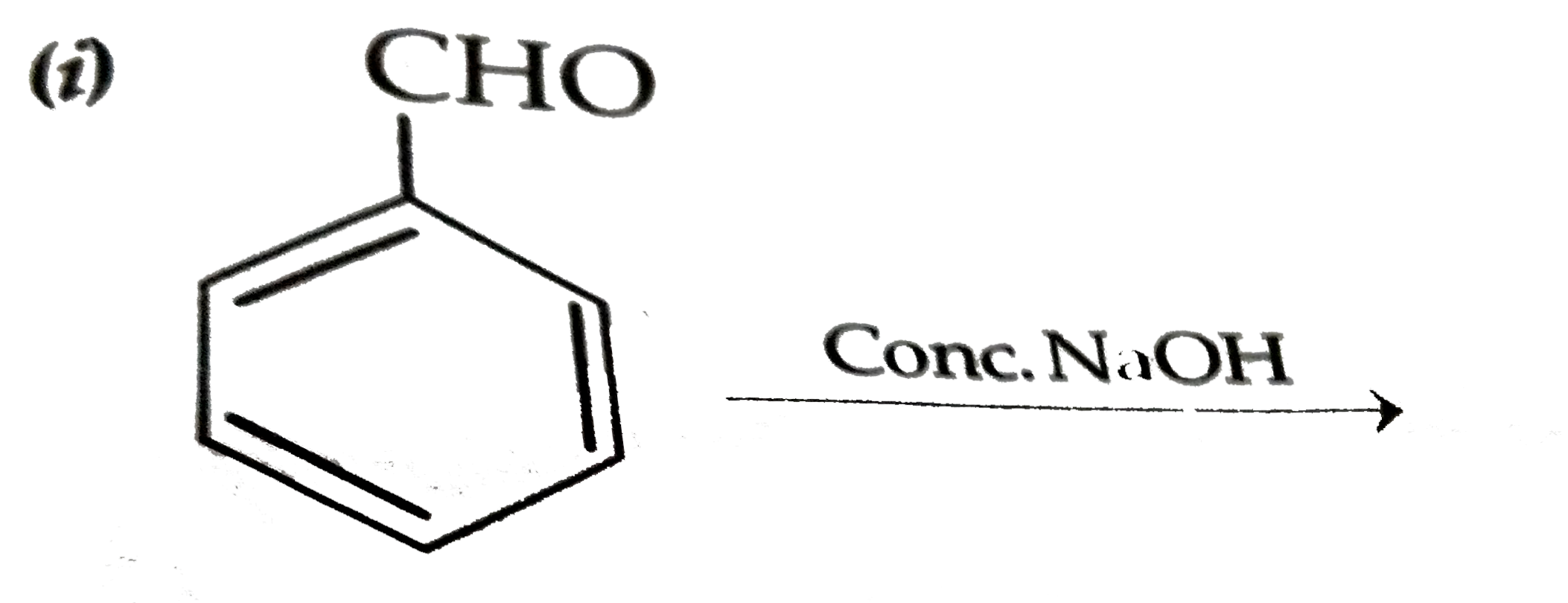
(ii) CH3COOHLiAIH4−−−−→
(iii) CH3−COOH3(a)Cl2/P−−−−−−→(b)H2O
(b) Distinguish between :
(ii) CH3−CHOandCH3−C∣∣O−CH3
View Solution(a) Write the reagent used in the following :
C6H5COCH3?−−→C6H5CH12CH3
(b) Arrange the following compounds in increasing order of reactivity towards nucleophilic addition.
CH3CHO,C6H5CHO,HCHO
View SolutionIdentify A and B in each of the following processes :
(i) CH3CH2NaCN−−−→Areduction−−−−→Ni/H2B
(ii) C6H5NH2NaNO2−−−−→AC6H5NH2−−−−−→OH−B
View SolutionPredict the products of the following reactions
(a)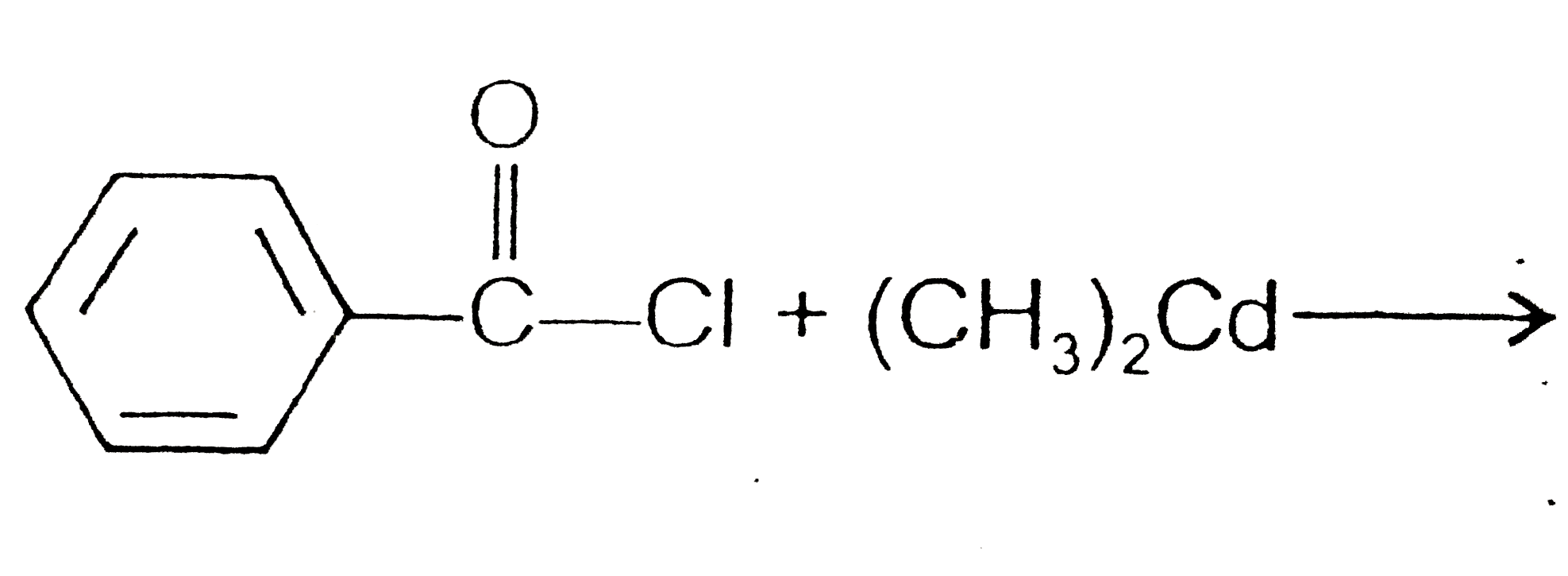
(b) CH3−O∣∣C−CH3(i)CH3MgBr−−−−−−−−→(ii)H3O+
(c )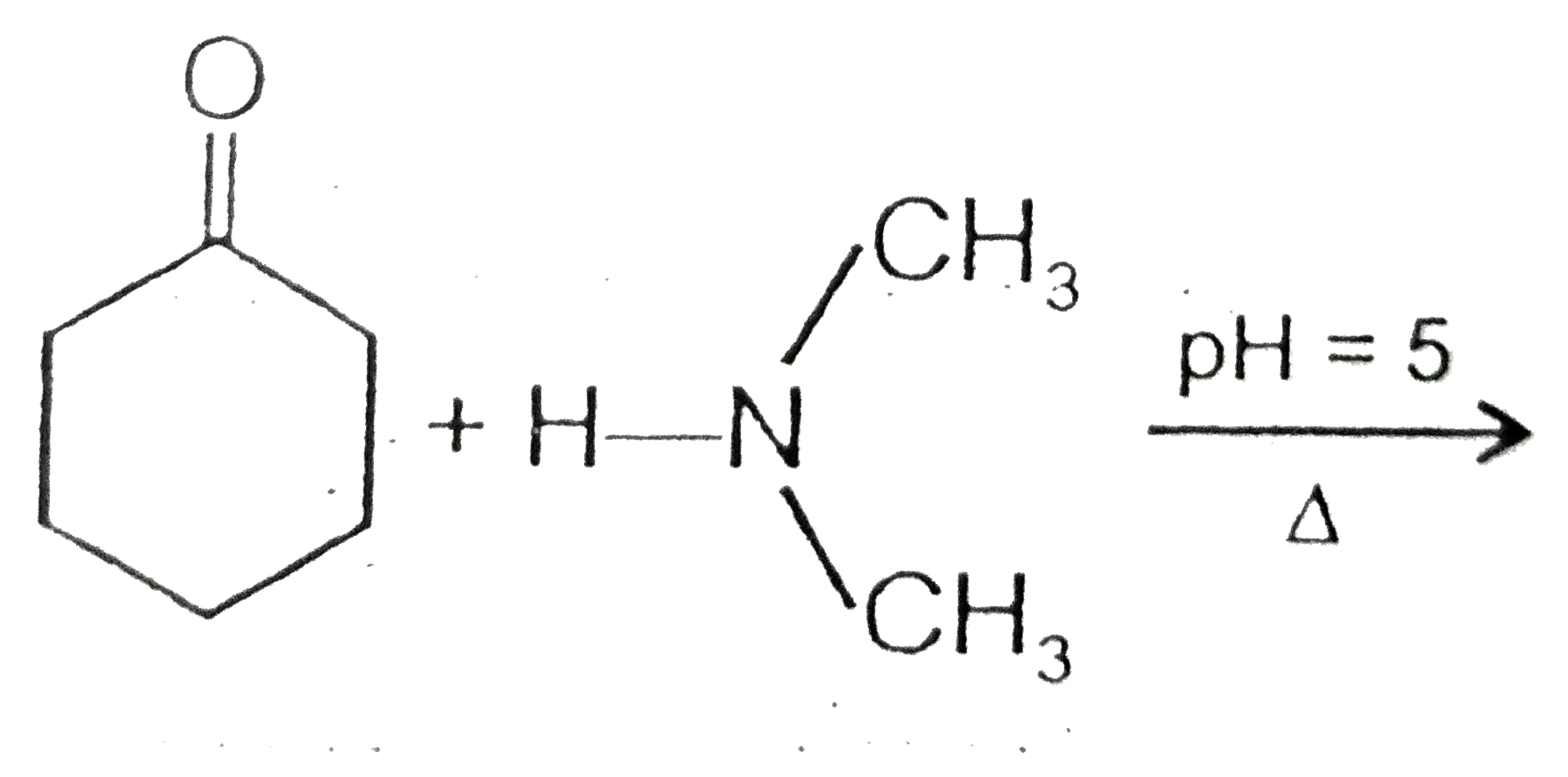
View Solution
