Write the decreasing order of
(a) carbon - carbon and
(b) carbon - hydrogen bond lengths in ethane, ethylene and acetylene molecules.
Video Solution
Text Solution
Carbon, carbon bond lengths are :
C2H6154 pm>C2H4 134 pm>C2H2120 pm
Carbon,hydrogen bond lengths are :
C2H6 100pm>C2H4109 pm>C2H2106pm
Calculation of no. of Hybrid orbitals and Pure orbitals in carbon compounds
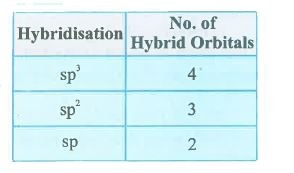
No. of pure orbitals =2(π) + No. of hydrogens
|
Similar Questions
Knowledge Check
The carbon - carbon bond length in graphite is
A1.34A0B1.54A0C1.42A0D1.20A0Carbon -halogen bond lengths increases in the order
AC−F<C−Cl<C−Br<C−IBC−I<C−Br<C−Cl<C−FCC−I<C−Br<C−F<C−ClDC−I<C−F<C−Br<C−ClThe carbon-carbon bond length in acetylene is
A120 pmB139 pmC154 pmD134 pm
The correct increasing order of carbon-carbon bond lengths in benzene, ethane, ethene, acetylene and graphite is :
View SolutionThe Carbon -Carbon bond length in diamond is
View SolutionBond length between carbon-carbon in ethylene molecule is
View SolutionWhat is the carbon-carbon bond length in benzene?
View SolutionCarbon-halogen bond lenghts increases in the order
View Solution
