The ration of pure orbitals to hybridized orbitals in ethylene is
2:3
3:1
1:1
1:3
The correct Answer is:C
Topper's Solved these Questions
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:NARAYNAChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:EXERCISE- II (C.W.) (DATIVE BOND)
Explore 2 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:NARAYNAChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:EXERCISE- II (C.W.) (MOLECULAR ORBITAL THEORY)
Explore 12 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:NARAYNAChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:EXERCISE- II (C.W.) (VSEPR THEORY)
Explore 7 Videos
- CARBONYL COMPOUNDSBook:NARAYNAChapter:CARBONYL COMPOUNDSExercise:LEVEL -VI
Explore 99 Videos
- CHEMICAL EQUILIBRIUMBook:NARAYNAChapter:CHEMICAL EQUILIBRIUMExercise:Exercise -IV
Explore 33 Videos
Similar Questions
Knowledge Check
Ratio of pure and hybrid orbitals in benzene
A3:2B2:3C1:2D2:1Number of sp2 hybrid orbitals in Benzene is
A18B24C6D12sp3d2 hybrid orbitals are
AlinearBpentagonal bipyramidalCtrigonal bipyramidalDoctahedral
Calculate the ratio between hybrid orbitals and pure orbitals in Allene.
CH2=C=CH2
View SolutionAs compared to pure atomic orbitals, hybrid orbitals have
View SolutionHybrid orbitals could make
View SolutionEthylene molecules is formed as a result of sp2 hybridisation of carbon. Each carbon atom is excited state undergo sp2 hybridisation giving rise to three hybrid orbitals each. These hybrid orbtals lie in the xy plane while the fourth unhybridised orbital lies at right angles to the hybridised orbitals. in the overlap ethylene two hybrid orbitals, i.e., one from each carbon atom from a sigma bond by head on overlap while the remaining overlap with hydrogen atoms. the unhybridised p-orbitals undergo sidewise overlap to from a π -bonds
Ground state of carbon atom
Excited state of carbon atom
The molecules of ethylene is planar
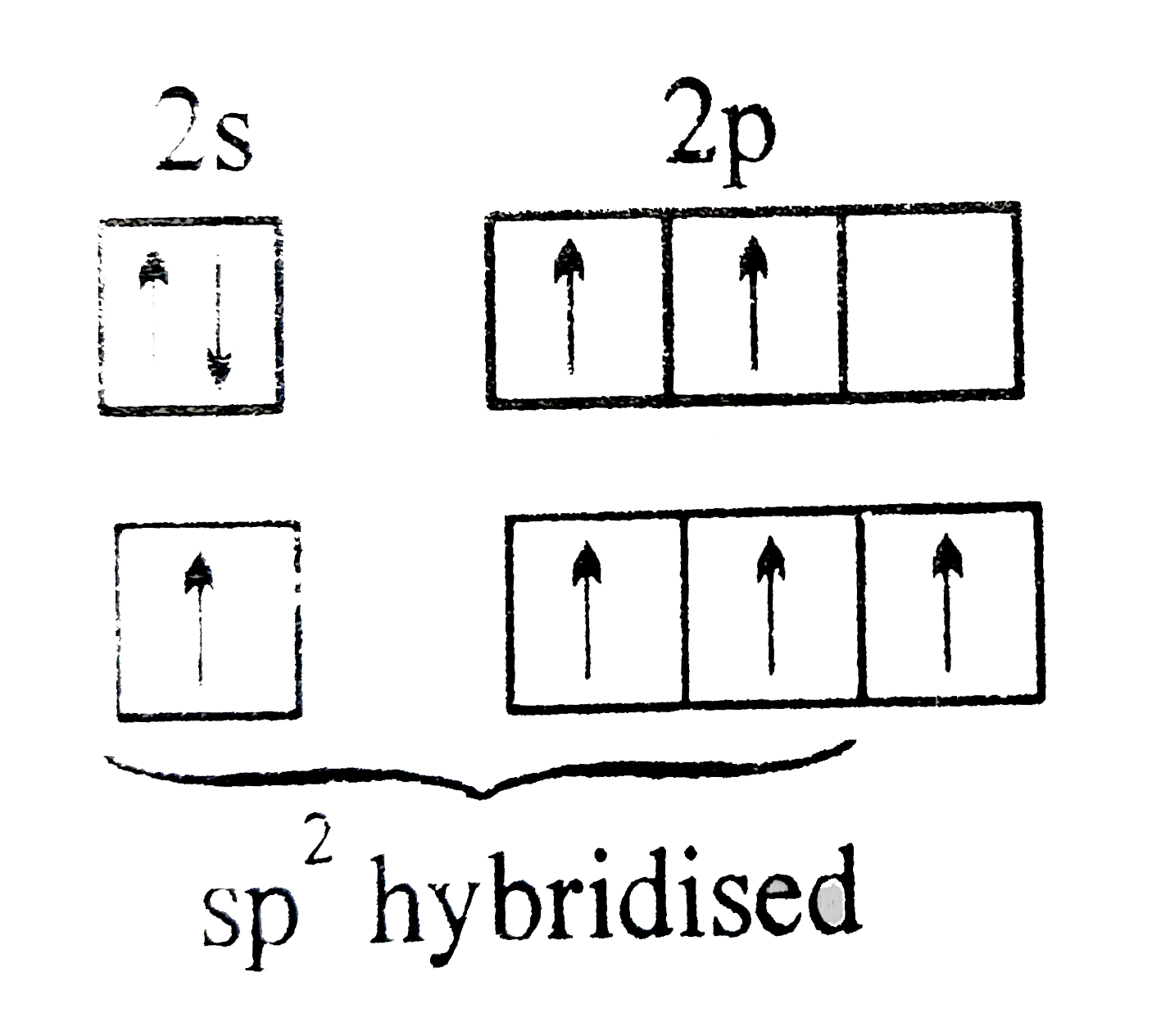
If molecular axis is z axis then
View SolutionEthylene molecules is formed as a result of sp2 hybridisation of carbon. Each carbon atom is excited state undergo sp2 hybridisation giving rise to three hybrid orbitals each. These hybrid orbtals lie in the xy plane while the fourth unhybridised orbital lies at right angles to the hybridised orbitals. in the overlap ethylene two hybrid orbitals, i.e., one from each carbon atom from a sigma bond by head on overlap while the remaining overlap with hydrogen atoms. the unhybridised p-orbitals undergo sidewise overlap to from a π -bonds
Ground state of carbon atom
Excited state of carbon atom
The molecules of ethylene is planar
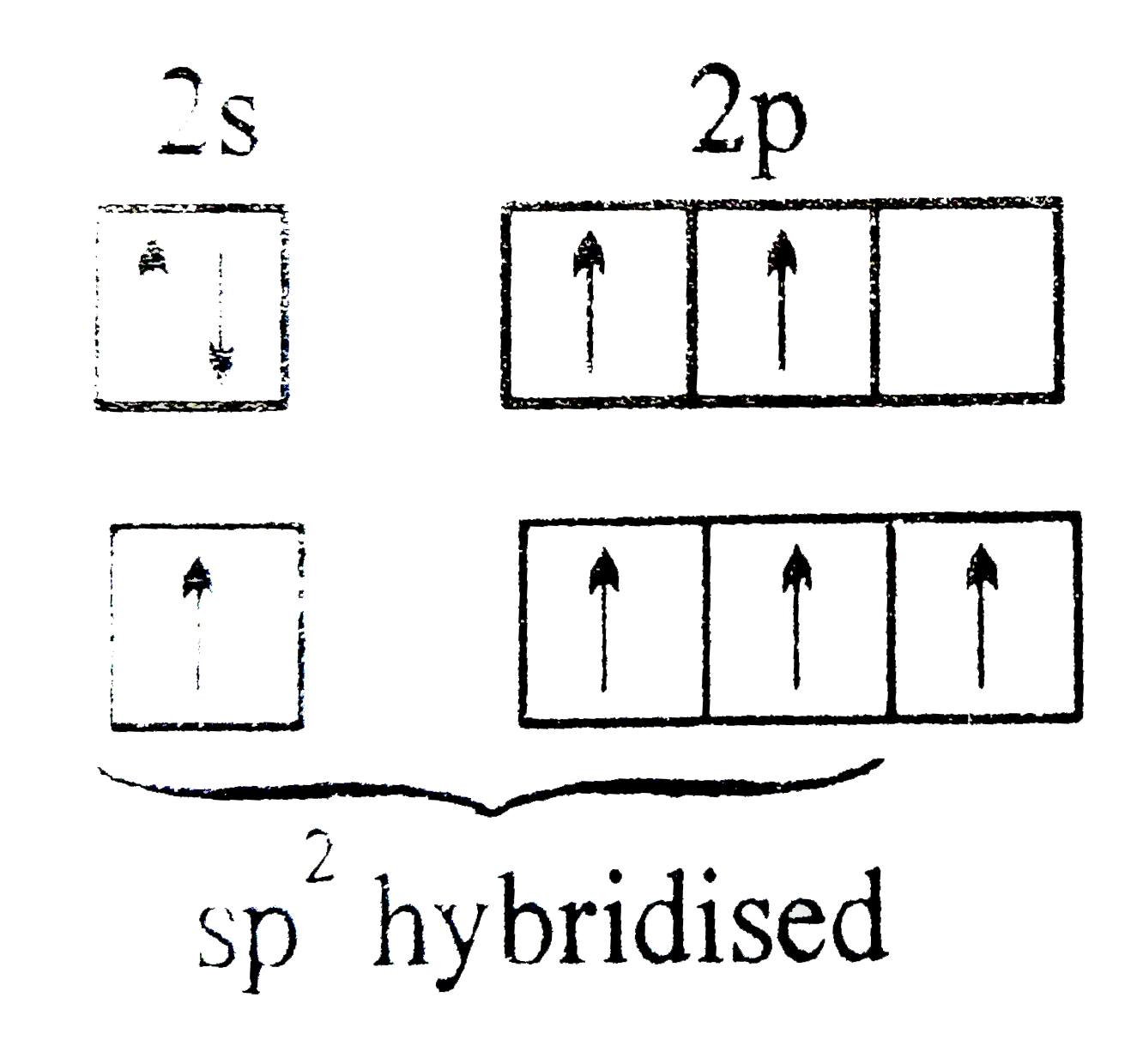
sp2 hybridised 'C' atoms are present in ethelene are
View Solution

